Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation
- PMID: 21697501
- PMCID: PMC3202898
- DOI: 10.1093/gerona/glr100
Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation
Abstract
Context: Testosterone in Older Men with Mobility Limitations Trial determined the effects of testosterone on muscle performance and physical function in older men with mobility limitation. Trial's Data and Safety Monitoring Board recommended enrollment cessation due to increased frequency of adverse events in testosterone arm. The changes in muscle performance and physical function were evaluated in relation to participant's perception of change.
Methods: Men aged 65 years and older, with mobility limitation, total testosterone 100-350 ng/dL, or free testosterone less than 50 pg/mL, were randomized to placebo or 10 g testosterone gel daily for 6 months. Primary outcome was leg-press strength. Secondary outcomes included chest-press strength, stair-climb, 40-m walk, muscle mass, physical activity, self-reported function, and fatigue. Proportions of participants exceeding minimally important difference in study arms were compared.
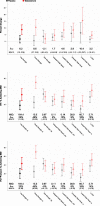
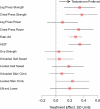
Results: Of 209 randomized participants, 165 had follow-up efficacy measures. Mean (SD) age was 74 (5.4) years and short physical performance battery score 7.7 (1.4). Testosterone arm exhibited greater improvements in leg-press strength, chest-press strength and power, and loaded stair-climb than placebo. Compared with placebo, significantly greater proportion of men receiving testosterone improved their leg-press and chest-press strengths (43% vs 18%, p = .01) and stair-climbing power (28% vs 10%, p = .03) more than minimally important difference. Increases in leg-press strength and stair-climbing power were associated with changes in testosterone levels and muscle mass. Physical activity, walking speed, self-reported function, and fatigue did not change.
Conclusions: Testosterone administration in older men with mobility limitation was associated with patient-important improvements in muscle strength and stair-climbing power. Improvements in muscle strength and only some physical function measures should be weighed against the risk of adverse events in this population.
Trial registration: ClinicalTrials.gov NCT00240981.
Figures
Similar articles
-
Effects of Testosterone Supplementation for 3 Years on Muscle Performance and Physical Function in Older Men.J Clin Endocrinol Metab. 2017 Feb 1;102(2):583-593. doi: 10.1210/jc.2016-2771. J Clin Endocrinol Metab. 2017. PMID: 27754805 Free PMC article. Clinical Trial.
-
Changes in muscle mass, muscle strength, and power but not physical function are related to testosterone dose in healthy older men.J Am Geriatr Soc. 2008 Nov;56(11):1991-9. doi: 10.1111/j.1532-5415.2008.01927.x. Epub 2008 Sep 15. J Am Geriatr Soc. 2008. PMID: 18795988 Free PMC article. Clinical Trial.
-
Effects of testosterone therapy on muscle performance and physical function in older men with mobility limitations (The TOM Trial): design and methods.Contemp Clin Trials. 2009 Mar;30(2):133-40. doi: 10.1016/j.cct.2008.10.005. Epub 2008 Oct 29. Contemp Clin Trials. 2009. PMID: 18996225 Free PMC article. Clinical Trial.
-
Hormone replacement therapy and physical function in healthy older men. Time to talk hormones?Endocr Rev. 2012 Jun;33(3):314-77. doi: 10.1210/er.2012-1002. Epub 2012 Mar 20. Endocr Rev. 2012. PMID: 22433122 Free PMC article. Review.
-
Androgens and Selective Androgen Receptor Modulators to Treat Functional Limitations Associated With Aging and Chronic Disease.J Gerontol A Biol Sci Med Sci. 2023 Jun 16;78(Suppl 1):25-31. doi: 10.1093/gerona/glad027. J Gerontol A Biol Sci Med Sci. 2023. PMID: 37325955 Free PMC article. Review.
Cited by
-
Selective Androgen Receptor Modulators as Function Promoting Therapies.J Frailty Aging. 2015;4(3):121-2. doi: 10.14283/jfa.2015.65. J Frailty Aging. 2015. PMID: 27030938 Free PMC article. No abstract available.
-
Muscular responses to testosterone replacement vary by administration route: a systematic review and meta-analysis.J Cachexia Sarcopenia Muscle. 2018 Jun;9(3):465-481. doi: 10.1002/jcsm.12291. Epub 2018 Mar 15. J Cachexia Sarcopenia Muscle. 2018. PMID: 29542875 Free PMC article.
-
Testosterone Replacement Therapy in Chronic Kidney Disease Patients.Nutrients. 2022 Aug 22;14(16):3444. doi: 10.3390/nu14163444. Nutrients. 2022. PMID: 36014950 Free PMC article.
-
The Interplay between Magnesium and Testosterone in Modulating Physical Function in Men.Int J Endocrinol. 2014;2014:525249. doi: 10.1155/2014/525249. Epub 2014 Mar 3. Int J Endocrinol. 2014. PMID: 24723948 Free PMC article. Review.
-
Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology-update 2014.J Cachexia Sarcopenia Muscle. 2014 Dec;5(4):253-9. doi: 10.1007/s13539-014-0161-y. Epub 2014 Oct 22. J Cachexia Sarcopenia Muscle. 2014. PMID: 25425503 Free PMC article.
References
-
- Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. - PubMed

