Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation
- PMID: 21695182
- PMCID: PMC3113798
- DOI: 10.1371/journal.pone.0019783
Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation
Erratum in
-
Correction: Mitochondrial Ceramide-Rich Macrodomains Functionalize Bax upon Irradiation.PLoS One. 2015 Dec 30;10(12):e0146210. doi: 10.1371/journal.pone.0146210. eCollection 2015. PLoS One. 2015. PMID: 26716446 Free PMC article. No abstract available.
Abstract
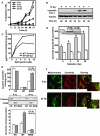
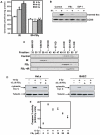
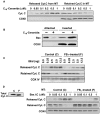
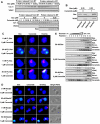
Background: Evidence indicates that Bax functions as a "lipidic" pore to regulate mitochondrial outer membrane permeabilization (MOMP), the apoptosis commitment step, through unknown membrane elements. Here we show mitochondrial ceramide elevation facilitates MOMP-mediated cytochrome c release in HeLa cells by generating a previously-unrecognized mitochondrial ceramide-rich macrodomain (MCRM), which we visualize and isolate, into which Bax integrates.
Methodology/principal findings: MCRMs, virtually non-existent in resting cells, form upon irradiation coupled to ceramide synthase-mediated ceramide elevation, optimizing Bax insertion/oligomerization and MOMP. MCRMs are detected by confocal microscopy in intact HeLa cells and isolated biophysically as a light membrane fraction from HeLa cell lysates. Inhibiting ceramide generation using a well-defined natural ceramide synthase inhibitor, Fumonisin B1, prevented radiation-induced Bax insertion, oligomerization and MOMP. MCRM deconstruction using purified mouse hepatic mitochondria revealed ceramide alone is non-apoptogenic. Rather Bax integrates into MCRMs, oligomerizing therein, conferring 1-2 log enhanced cytochrome c release. Consistent with this mechanism, MCRM Bax isolates as high molecular weight "pore-forming" oligomers, while non-MCRM membrane contains exclusively MOMP-incompatible monomeric Bax.
Conclusions/significance: Our recent studies in the C. elegans germline indicate that mitochondrial ceramide generation is obligate for radiation-induced apoptosis, although a mechanism for ceramide action was not delineated. Here we demonstrate that ceramide, generated in the mitochondrial outer membrane of mammalian cells upon irradiation, forms a platform into which Bax inserts, oligomerizes and functionalizes as a pore. We posit conceptualization of ceramide as a membrane-based stress calibrator, driving membrane macrodomain organization, which in mitochondria regulates intensity of Bax-induced MOMP, and is pharmacologically tractable in vitro and in vivo.
Conflict of interest statement
Figures
Similar articles
-
Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane.Apoptosis. 2010 May;15(5):553-62. doi: 10.1007/s10495-009-0449-0. Apoptosis. 2010. PMID: 20101465
-
The BCL-2 protein BAK is required for long-chain ceramide generation during apoptosis.J Biol Chem. 2010 Apr 16;285(16):11818-26. doi: 10.1074/jbc.M109.078121. Epub 2010 Feb 18. J Biol Chem. 2010. PMID: 20172858 Free PMC article.
-
BAX insertion, oligomerization, and outer membrane permeabilization in brain mitochondria: role of permeability transition and SH-redox regulation.Biochim Biophys Acta. 2010 Nov;1797(11):1795-806. doi: 10.1016/j.bbabio.2010.07.006. Epub 2010 Jul 23. Biochim Biophys Acta. 2010. PMID: 20655869 Free PMC article.
-
Regulation of Bax mitochondrial localization by Bcl-2 and Bcl-x(L): keep your friends close but your enemies closer.Int J Biochem Cell Biol. 2013 Jan;45(1):64-7. doi: 10.1016/j.biocel.2012.09.022. Epub 2012 Oct 11. Int J Biochem Cell Biol. 2013. PMID: 23064052 Review.
-
Ceramide channels and mitochondrial outer membrane permeability.J Bioenerg Biomembr. 2017 Feb;49(1):57-64. doi: 10.1007/s10863-016-9646-z. Epub 2016 Jan 22. J Bioenerg Biomembr. 2017. PMID: 26801188 Review.
Cited by
-
Metabolic Regulation of Apoptosis in Cancer.Int Rev Cell Mol Biol. 2016;327:43-87. doi: 10.1016/bs.ircmb.2016.06.006. Epub 2016 Jul 30. Int Rev Cell Mol Biol. 2016. PMID: 27692180 Free PMC article. Review.
-
Regulatory Role of Sphingosine-1-Phosphate and C16:0 Ceramide, in Immunogenic Cell Death of Colon Cancer Cells Induced by Bak/Bax-Activation.Cancers (Basel). 2022 Oct 22;14(21):5182. doi: 10.3390/cancers14215182. Cancers (Basel). 2022. PMID: 36358599 Free PMC article.
-
A search for ceramide binding proteins using bifunctional lipid analogs yields CERT-related protein StarD7.J Lipid Res. 2018 Mar;59(3):515-530. doi: 10.1194/jlr.M082354. Epub 2018 Jan 17. J Lipid Res. 2018. PMID: 29343537 Free PMC article.
-
Ceramide synthase inhibitor fumonisin B1 inhibits apoptotic cell death in SCC17B human head and neck squamous carcinoma cells after Pc4 photosensitization.Photochem Photobiol Sci. 2014 Nov;13(11):1621-7. doi: 10.1039/c4pp00292j. Photochem Photobiol Sci. 2014. PMID: 25266739 Free PMC article.
-
Regulation of ceramide channel formation and disassembly: Insights on the initiation of apoptosis.Saudi J Biol Sci. 2015 Nov;22(6):760-72. doi: 10.1016/j.sjbs.2015.03.005. Epub 2015 Mar 22. Saudi J Biol Sci. 2015. PMID: 26587005 Free PMC article.
References
-
- Roucou X, Martinou JC. Conformational change of Bax: a question of life or death. Cell Death Differ. 2001;8:875–877. - PubMed
-
- Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. - PubMed
-
- Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. - PubMed
-
- Garcia-Saez AJ, Mingarro I, Perez-Paya E, Salgado J. Membrane-insertion fragments of Bcl-xL, Bax, and Bid. Biochemistry. 2004;43:10930–10943. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

