Sprouty genes prevent excessive FGF signalling in multiple cell types throughout development of the cerebellum
- PMID: 21693512
- PMCID: PMC3119305
- DOI: 10.1242/dev.063784
Sprouty genes prevent excessive FGF signalling in multiple cell types throughout development of the cerebellum
Abstract
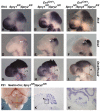
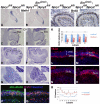
Fibroblast growth factors (FGFs) and regulators of the FGF signalling pathway are expressed in several cell types within the cerebellum throughout its development. Although much is known about the function of this pathway during the establishment of the cerebellar territory during early embryogenesis, the role of this pathway during later developmental stages is still poorly understood. Here, we investigated the function of sprouty genes (Spry1, Spry2 and Spry4), which encode feedback antagonists of FGF signalling, during cerebellar development in the mouse. Simultaneous deletion of more than one of these genes resulted in a number of defects, including mediolateral expansion of the cerebellar vermis, reduced thickness of the granule cell layer and abnormal foliation. Analysis of cerebellar development revealed that the anterior cerebellar neuroepithelium in the early embryonic cerebellum was expanded and that granule cell proliferation during late embryogenesis and early postnatal development was reduced. We show that the granule cell proliferation deficit correlated with reduced sonic hedgehog (SHH) expression and signalling. A reduction in Fgfr1 dosage during development rescued these defects, confirming that the abnormalities are due to excess FGF signalling. Our data indicate that sprouty acts both cell autonomously in granule cell precursors and non-cell autonomously to regulate granule cell number. Taken together, our data demonstrate that FGF signalling levels have to be tightly controlled throughout cerebellar development in order to maintain the normal development of multiple cell types.
Figures






Similar articles
-
Spry1 and Spry2 are necessary for eyelid closure.Dev Biol. 2013 Nov 15;383(2):227-38. doi: 10.1016/j.ydbio.2013.09.014. Epub 2013 Sep 17. Dev Biol. 2013. PMID: 24055172 Free PMC article.
-
Coordinated activity of Spry1 and Spry2 is required for normal development of the external genitalia.Dev Biol. 2014 Feb 1;386(1):1-11. doi: 10.1016/j.ydbio.2013.12.014. Epub 2013 Dec 18. Dev Biol. 2014. PMID: 24361260 Free PMC article.
-
Cosuppression of Sprouty and Sprouty-related negative regulators of FGF signalling in prostate cancer: a working hypothesis.Biomed Res Int. 2015;2015:827462. doi: 10.1155/2015/827462. Epub 2015 May 17. Biomed Res Int. 2015. PMID: 26075267 Free PMC article.
-
FGF signalling: diverse roles during early vertebrate embryogenesis.Development. 2010 Nov;137(22):3731-42. doi: 10.1242/dev.037689. Development. 2010. PMID: 20978071 Free PMC article. Review.
-
Regulation of cerebellar network development by granule cells and their molecules.Front Mol Neurosci. 2023 Jul 14;16:1236015. doi: 10.3389/fnmol.2023.1236015. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37520428 Free PMC article. Review.
Cited by
-
The Fibroblast Growth Factor signaling pathway.Wiley Interdiscip Rev Dev Biol. 2015 May-Jun;4(3):215-66. doi: 10.1002/wdev.176. Epub 2015 Mar 13. Wiley Interdiscip Rev Dev Biol. 2015. PMID: 25772309 Free PMC article. Review.
-
Sprouty3 and Sprouty4, Two Members of a Family Known to Inhibit FGF-Mediated Signaling, Exert Opposing Roles on Proliferation and Migration of Glioblastoma-Derived Cells.Cells. 2019 Aug 1;8(8):808. doi: 10.3390/cells8080808. Cells. 2019. PMID: 31374860 Free PMC article.
-
Secretome Screening Reveals Fibroblast Growth Factors as Novel Inhibitors of Viral Replication.J Virol. 2018 Jul 31;92(16):e00260-18. doi: 10.1128/JVI.00260-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29899088 Free PMC article.
-
Sprouty is a negative regulator of transforming growth factor β-induced epithelial-to-mesenchymal transition and cataract.Mol Med. 2012 Jul 18;18(1):861-73. doi: 10.2119/molmed.2012.00111. Mol Med. 2012. PMID: 22517312 Free PMC article.
-
Submandibular parasympathetic gangliogenesis requires sprouty-dependent Wnt signals from epithelial progenitors.Dev Cell. 2015 Mar 23;32(6):667-77. doi: 10.1016/j.devcel.2015.01.023. Dev Cell. 2015. PMID: 25805134 Free PMC article.
References
-
- Baptista C. A., Hatten M. E., Blazeski R., Mason C. A. (1994). Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro. Neuron 12, 243-260 - PubMed
-
- Basson M. A., Akbulut S., Watson-Johnson J., Simon R., Carroll T. J., Shakya R., Gross I., Martin G. R., Lufkin T., McMahon A. P., et al. (2005). Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev. Cell 8, 229-239 - PubMed
-
- Berry M., Bradley P. (1976). The growth of the dendritic trees of Purkinje cells in irradiated agranular cerebellar cortex. Brain Res. 116, 361-387 - PubMed
-
- Bizzoca A., Virgintino D., Lorusso L., Buttiglione M., Yoshida L., Polizzi A., Tattoli M., Cagiano R., Rossi F., Kozlov S., et al. (2003). Transgenic mice expressing F3/contactin from the TAG-1 promoter exhibit developmentally regulated changes in the differentiation of cerebellar neurons. Development 130, 29-43 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

