Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody
- PMID: 21690411
- PMCID: PMC3131343
- DOI: 10.1073/pnas.1103012108
Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody
Abstract
To guide vaccine design, we assessed whether human monoclonal antibodies (MAbs) b12 and b6 against the CD4 binding site (CD4bs) on HIV-1 gp120 and F240 against an immundominant epitope on gp41 could prevent vaginal transmission of simian HIV (SHIV)-162P4 to macaques. The two anti-gp120 MAbs have similar monomeric gp120-binding properties, measured in vitro, but b12 is strongly neutralizing and b6 is not. F240 is nonneutralizing. Applied vaginally at a high dose, the strongly neutralizing MAb b12 provided sterilizing immunity in seven of seven animals, b6 in zero of five animals, and F240 in two of five animals. Compared with control animals, the protection by b12 achieved statistical significance, whereas that caused by F240 did not. For two of three unprotected F240-treated animals there was a trend toward lowered viremia. The potential protective effect of F240 may relate to the relatively strong ability of this antibody to capture infectious virions. Additional passive transfer experiments also indicated that the ability of the administered anti-gp120 MAbs to neutralize the challenge virus was a critical influence on protection. Furthermore, when data from all of the experiments were combined, there was a significant increase in the number of founder viruses establishing infection in animals receiving MAb b6, compared with other nonprotected macaques. Thus, a gp120-binding, weakly neutralizing MAb to the CD4bs was, at best, completely ineffective at protection. A nonneutralizing antibody to gp41 may have a limited capacity to protect, but the results suggest that the central focus of HIV-1 vaccine research should be on the induction of potently neutralizing antibodies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
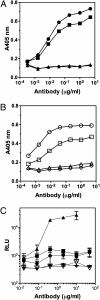


Similar articles
-
Protection of neonatal macaques against experimental SHIV infection by human neutralizing monoclonal antibodies.Transfus Clin Biol. 2001 Aug;8(4):350-8. doi: 10.1016/s1246-7820(01)00187-2. Transfus Clin Biol. 2001. PMID: 11642027 Review.
-
Development of Broadly Neutralizing Antibodies and Their Mapping by Monomeric gp120 in Human Immunodeficiency Virus Type 1-Infected Humans and Simian-Human Immunodeficiency Virus SHIVSF162P3N-Infected Macaques.J Virol. 2016 Mar 28;90(8):4017-4031. doi: 10.1128/JVI.02898-15. Print 2016 Apr. J Virol. 2016. PMID: 26842476 Free PMC article.
-
Induction of HIV-specific antibody response and protection against vaginal SHIV transmission by intranasal immunization with inactivated SHIV-capturing nanospheres in macaques.J Med Virol. 2004 Jul;73(3):368-77. doi: 10.1002/jmv.20100. J Med Virol. 2004. PMID: 15170630
-
Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers.PLoS Pathog. 2009 May;5(5):e1000433. doi: 10.1371/journal.ppat.1000433. Epub 2009 May 15. PLoS Pathog. 2009. PMID: 19436712 Free PMC article.
-
Passive immunization with human neutralizing monoclonal antibodies: correlates of protective immunity against HIV.Vaccine. 2002 May 6;20(15):1956-60. doi: 10.1016/s0264-410x(02)00077-4. Vaccine. 2002. PMID: 11983253 Review.
Cited by
-
Short Communication: Virion Aggregation by Neutralizing and Nonneutralizing Antibodies to the HIV-1 Envelope Glycoprotein.AIDS Res Hum Retroviruses. 2015 Nov;31(11):1160-5. doi: 10.1089/AID.2015.0050. Epub 2015 Jul 20. AIDS Res Hum Retroviruses. 2015. PMID: 26086186 Free PMC article.
-
Adjuvanting a Simian Immunodeficiency Virus Vaccine with Toll-Like Receptor Ligands Encapsulated in Nanoparticles Induces Persistent Antibody Responses and Enhanced Protection in TRIM5α Restrictive Macaques.J Virol. 2017 Jan 31;91(4):e01844-16. doi: 10.1128/JVI.01844-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27928002 Free PMC article.
-
Elimination of SHIV Infected Cells by Combinations of Bispecific HIVxCD3 DART® Molecules.Front Immunol. 2021 Aug 13;12:710273. doi: 10.3389/fimmu.2021.710273. eCollection 2021. Front Immunol. 2021. PMID: 34484212 Free PMC article.
-
The importance of semen leukocytes in HIV-1 transmission and the development of prevention strategies.Hum Vaccin Immunother. 2020 Sep 1;16(9):2018-2032. doi: 10.1080/21645515.2020.1765622. Epub 2020 Jul 2. Hum Vaccin Immunother. 2020. PMID: 32614649 Free PMC article. Review.
-
Lessons learned from HIV vaccine clinical efficacy trials.Curr HIV Res. 2013 Sep;11(6):441-9. doi: 10.2174/1570162x113116660051. Curr HIV Res. 2013. PMID: 24033299 Free PMC article. Review.
References
-
- Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413–444. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

