Measuring naturally acquired immune responses to candidate malaria vaccine antigens in Ghanaian adults
- PMID: 21689436
- PMCID: PMC3132199
- DOI: 10.1186/1475-2875-10-168
Measuring naturally acquired immune responses to candidate malaria vaccine antigens in Ghanaian adults
Abstract
Background: To prepare field sites for malaria vaccine trials, it is important to determine baseline antibody and T cell responses to candidate malaria vaccine antigens. Assessing T cell responses is especially challenging, given genetic restriction, low responses observed in endemic areas, their variability over time, potential suppression by parasitaemia and the intrinsic variability of the assays.
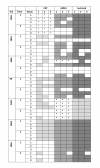
Methods: In Part A of this study, antibody titres were measured in adults from urban and rural communities in Ghana to recombinant Plasmodium falciparum CSP, SSP2/TRAP, LSA1, EXP1, MSP1, MSP3 and EBA175 by ELISA, and to sporozoites and infected erythrocytes by IFA. Positive ELISA responses were determined using two methods. T cell responses to defined CD8 or CD4 T cell epitopes from CSP, SSP2/TRAP, LSA1 and EXP1 were measured by ex vivo IFN-γ ELISpot assays using HLA-matched Class I- and DR-restricted synthetic peptides. In Part B, the reproducibility of the ELISpot assay to CSP and AMA1 was measured by repeating assays of individual samples using peptide pools and low, medium or high stringency criteria for defining positive responses, and by comparing samples collected two weeks apart.
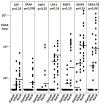
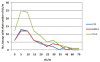
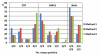
Results: In Part A, positive antibody responses varied widely from 17%-100%, according to the antigen and statistical method, with blood stage antigens showing more frequent and higher magnitude responses. ELISA titres were higher in rural subjects, while IFA titres and the frequencies and magnitudes of ex vivo ELISpot activities were similar in both communities. DR-restricted peptides showed stronger responses than Class I-restricted peptides. In Part B, the most stringent statistical criteria gave the fewest, and the least stringent the most positive responses, with reproducibility slightly higher using the least stringent method when assays were repeated. Results varied significantly between the two-week time-points for many participants.
Conclusions: All participants were positive for at least one malaria protein by ELISA, with results dependent on the criteria for positivity. Likewise, ELISpot responses varied among participants, but were relatively reproducible by the three methods tested, especially the least stringent, when assays were repeated. However, results often differed between samples taken two weeks apart, indicating significant biological variability over short intervals.
Figures



Similar articles
-
Measurement of ex vivo ELISpot interferon-gamma recall responses to Plasmodium falciparum AMA1 and CSP in Ghanaian adults with natural exposure to malaria.Malar J. 2016 Feb 1;15:55. doi: 10.1186/s12936-016-1098-8. Malar J. 2016. PMID: 26830334 Free PMC article.
-
Measuring naturally acquired ex vivo IFN-γ responses to Plasmodium falciparum cell-traversal protein for ookinetes and sporozoites (CelTOS) in Ghanaian adults.Malar J. 2015 Jan 21;14:20. doi: 10.1186/s12936-014-0539-5. Malar J. 2015. PMID: 25604473 Free PMC article.
-
Human adenovirus 5-vectored Plasmodium falciparum NMRC-M3V-Ad-PfCA vaccine encoding CSP and AMA1 is safe, well-tolerated and immunogenic but does not protect against controlled human malaria infection.Hum Vaccin Immunother. 2013 Oct;9(10):2165-77. doi: 10.4161/hv.24941. Epub 2013 Jun 4. Hum Vaccin Immunother. 2013. PMID: 23899517 Free PMC article. Clinical Trial.
-
Comprehensive Review of Human Plasmodium falciparum-Specific CD8+ T Cell Epitopes.Front Immunol. 2019 Mar 21;10:397. doi: 10.3389/fimmu.2019.00397. eCollection 2019. Front Immunol. 2019. PMID: 30949162 Free PMC article. Review.
-
Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens.Front Immunol. 2020 Feb 21;11:190. doi: 10.3389/fimmu.2020.00190. eCollection 2020. Front Immunol. 2020. PMID: 32153565 Free PMC article. Review.
Cited by
-
The Threshold of Protection from Liver-Stage Malaria Relies on a Fine Balance between the Number of Infected Hepatocytes and Effector CD8+ T Cells Present in the Liver.J Immunol. 2017 Mar 1;198(5):2006-2016. doi: 10.4049/jimmunol.1601209. Epub 2017 Jan 13. J Immunol. 2017. PMID: 28087668 Free PMC article.
-
Synthetic Antigens Derived from Plasmodium falciparum Sporozoite, Liver, and Blood Stages: Naturally Acquired Immune Response and Human Leukocyte Antigen Associations in Individuals Living in a Brazilian Endemic Area.Am J Trop Med Hyg. 2017 Nov;97(5):1581-1592. doi: 10.4269/ajtmh.17-0359. Epub 2017 Oct 10. Am J Trop Med Hyg. 2017. PMID: 29016339 Free PMC article.
-
Identifying Plasmodium P36 and P52 antigens for co-administration with circumsporozoite protein to enhance vaccine efficacy.Res Sq [Preprint]. 2024 Sep 24:rs.3.rs-4909396. doi: 10.21203/rs.3.rs-4909396/v1. Res Sq. 2024. Update in: NPJ Vaccines. 2024 Dec 6;9(1):241. doi: 10.1038/s41541-024-01040-6 PMID: 39399676 Free PMC article. Updated. Preprint.
-
Measurement of ex vivo ELISpot interferon-gamma recall responses to Plasmodium falciparum AMA1 and CSP in Ghanaian adults with natural exposure to malaria.Malar J. 2016 Feb 1;15:55. doi: 10.1186/s12936-016-1098-8. Malar J. 2016. PMID: 26830334 Free PMC article.
-
A statistical interaction between circumsporozoite protein-specific T cell and antibody responses and risk of clinical malaria episodes following vaccination with RTS,S/AS01E.PLoS One. 2012;7(12):e52870. doi: 10.1371/journal.pone.0052870. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300801 Free PMC article.
References
-
- Todryk SM, Bejon P, Mwangi T, Plebanski M, Urban B, Marsh K, Hill AV, Flanagan KL. Correlation of memory T cell responses against TRAP with protection from clinical malaria, and CD4 CD25 high T cells with susceptibility in Kenyans. PLoS ONE. 2008;3:e2027. doi: 10.1371/journal.pone.0002027. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

