Heteromerization of human cytomegalovirus encoded chemokine receptors
- PMID: 21684267
- PMCID: PMC3156895
- DOI: 10.1016/j.bcp.2011.06.009
Heteromerization of human cytomegalovirus encoded chemokine receptors
Abstract
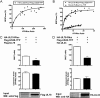
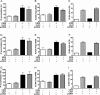
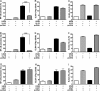
Human cytomegalovirus (HCMV) is a widespread pathogen that infects up to 80% of the human population and causes severe complications in immunocompromised patients. HCMV expresses four seven transmembrane (7TM) spanning/G protein-coupled receptors (GPCRs) - US28, US27, UL33 and UL78 - that show close homology to human chemokine receptors. While US28 was shown to bind several chemokines and to constitutively activate multiple signaling cascades, the function(s) of US27, UL33 and UL78 in the viral life cycle have not yet been identified. Here we investigated the possible interaction/heteromerization of US27, UL33 and UL78 with US28 and the functional consequences thereof. We provide evidence that these receptors not only co-localize, but also heteromerize with US28 in vitro. While the constitutive activation of the US28-mediated Gαq/phospholipase C pathway was not affected by receptor heteromerization, UL33 and UL78 were able to silence US28-mediated activation of the transcription factor NF-κB. Summarized, we provide evidence that these orphan viral receptors have an important regulatory capacity on the function of US28 and as a consequence, may ultimately impact on the viral life cycle of HCMV.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
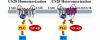


Similar articles
-
The Human Cytomegalovirus US27 Gene Product Constitutively Activates Antioxidant Response Element-Mediated Transcription through Gβγ, Phosphoinositide 3-Kinase, and Nuclear Respiratory Factor 1.J Virol. 2018 Nov 12;92(23):e00644-18. doi: 10.1128/JVI.00644-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30209167 Free PMC article.
-
Human cytomegalovirus-encoded UL33 and UL78 heteromerize with host CCR5 and CXCR4 impairing their HIV coreceptor activity.Blood. 2012 May 24;119(21):4908-18. doi: 10.1182/blood-2011-08-372516. Epub 2012 Apr 10. Blood. 2012. PMID: 22496149
-
Human Cytomegalovirus-Encoded G Protein-Coupled Receptor UL33 Facilitates Virus Dissemination via the Extracellular and Cell-to-Cell Route.Viruses. 2020 May 30;12(6):594. doi: 10.3390/v12060594. Viruses. 2020. PMID: 32486172 Free PMC article.
-
The HCMV chemokine receptor US28 is a potential target in vascular disease.Curr Drug Targets Infect Disord. 2001 Aug;1(2):151-8. doi: 10.2174/1568005014606080. Curr Drug Targets Infect Disord. 2001. PMID: 12455411 Review.
-
Human Cytomegalovirus US28: a functionally selective chemokine binding receptor.Infect Disord Drug Targets. 2009 Nov;9(5):548-56. doi: 10.2174/187152609789105696. Infect Disord Drug Targets. 2009. PMID: 19594424 Free PMC article. Review.
Cited by
-
Identification of common mechanisms by which human and mouse cytomegalovirus seven-transmembrane receptor homologues contribute to in vivo phenotypes in a mouse model.J Virol. 2013 Apr;87(7):4112-7. doi: 10.1128/JVI.03406-12. Epub 2013 Jan 23. J Virol. 2013. PMID: 23345521 Free PMC article.
-
CXCR4: a virus's best friend?Infect Genet Evol. 2014 Jul;25:146-56. doi: 10.1016/j.meegid.2014.04.018. Epub 2014 May 2. Infect Genet Evol. 2014. PMID: 24793563 Free PMC article. Review.
-
Insights into the Transcriptome of Human Cytomegalovirus: A Comprehensive Review.Viruses. 2023 Aug 8;15(8):1703. doi: 10.3390/v15081703. Viruses. 2023. PMID: 37632045 Free PMC article. Review.
-
Hunting for the function of orphan GPCRs - beyond the search for the endogenous ligand.Br J Pharmacol. 2015 Jul;172(13):3212-28. doi: 10.1111/bph.12942. Epub 2014 Dec 15. Br J Pharmacol. 2015. PMID: 25231237 Free PMC article. Review.
-
Orphan GPR61, GPR62 and GPR135 receptors and the melatonin MT2 receptor reciprocally modulate their signaling functions.Sci Rep. 2017 Aug 21;7(1):8990. doi: 10.1038/s41598-017-08996-7. Sci Rep. 2017. PMID: 28827538 Free PMC article.
References
-
- Humar A., Michaels M. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6(2):262–274. - PubMed
-
- Gandhi M.K., Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis. 2004;4(12):725–738. - PubMed
-
- Ross S.A., Boppana S.B. Congenital cytomegalovirus infection: outcome and diagnosis. Semin Pediatr Infect Dis. 2005;16(1):44–49. - PubMed
-
- Griffiths P.D. CMV as a cofactor enhancing progression of AIDS. J Clin Virol. 2006;35(4):489–492. - PubMed
-
- Rosenkilde M.M., Waldhoer M., Luttichau H.R., Schwartz T.W. Virally encoded 7TM receptors. Oncogene. 2001;20(13):1582–1593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

