Evidence of a role for soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) machinery in HIV-1 assembly and release
- PMID: 21680744
- PMCID: PMC3191027
- DOI: 10.1074/jbc.M111.241521
Evidence of a role for soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) machinery in HIV-1 assembly and release
Abstract
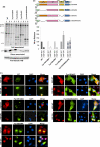
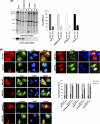
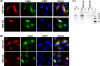
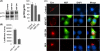
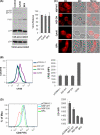
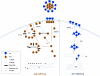
Retrovirus assembly is a complex process that requires the orchestrated participation of viral components and host-cell factors. The concerted movement of different viral proteins to specific sites in the plasma membrane allows for virus particle assembly and ultimately budding and maturation of infectious virions. The soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins constitute the minimal machinery that catalyzes the fusion of intracellular vesicles with the plasma membrane, thus regulating protein trafficking. Using siRNA and dominant negative approaches we demonstrate here that generalized disruption of the host SNARE machinery results in a significant reduction in human immunodeficiency virus type 1 (HIV-1) and equine infectious anemia virus particle production. Further analysis of the mechanism involved revealed a defect at the level of HIV-1 Gag localization to the plasma membrane. Our findings demonstrate for the first time a role of SNARE proteins in HIV-1 assembly and release, likely by affecting cellular trafficking pathways required for Gag transport and association with the plasma membrane.
Figures
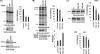
Similar articles
-
Roles of Cellular NSF Protein in Entry and Nuclear Egress of Budded Virions of Autographa californica Multiple Nucleopolyhedrovirus.J Virol. 2017 Sep 27;91(20):e01111-17. doi: 10.1128/JVI.01111-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28747507 Free PMC article.
-
Essential and supporting host cell factors for HIV-1 budding.Future Microbiol. 2011 Oct;6(10):1159-70. doi: 10.2217/fmb.11.100. Future Microbiol. 2011. PMID: 22004035 Review.
-
Budding of a Retrovirus: Some Assemblies Required.Viruses. 2020 Oct 20;12(10):1188. doi: 10.3390/v12101188. Viruses. 2020. PMID: 33092109 Free PMC article. Review.
-
Intracellular trafficking of HIV-1 Gag via Syntaxin 6-positive compartments/vesicles: Involvement in tumor necrosis factor secretion.J Biol Chem. 2024 Mar;300(3):105687. doi: 10.1016/j.jbc.2024.105687. Epub 2024 Jan 26. J Biol Chem. 2024. PMID: 38280430 Free PMC article.
-
Subcellular Localization of HIV-1 gag-pol mRNAs Regulates Sites of Virion Assembly.J Virol. 2017 Feb 28;91(6):e02315-16. doi: 10.1128/JVI.02315-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28053097 Free PMC article.
Cited by
-
Subtype Differences in the Interaction of HIV-1 Matrix with Calmodulin: Implications for Biological Functions.Biomolecules. 2021 Aug 31;11(9):1294. doi: 10.3390/biom11091294. Biomolecules. 2021. PMID: 34572507 Free PMC article.
-
Human Ubc9 is involved in intracellular HIV-1 Env stability after trafficking out of the trans-Golgi network in a Gag dependent manner.PLoS One. 2013 Jul 8;8(7):e69359. doi: 10.1371/journal.pone.0069359. Print 2013. PLoS One. 2013. PMID: 23861967 Free PMC article.
-
HIV Assembly and Budding: Ca(2+) Signaling and Non-ESCRT Proteins Set the Stage.Mol Biol Int. 2012;2012:851670. doi: 10.1155/2012/851670. Epub 2012 Jun 12. Mol Biol Int. 2012. PMID: 22761998 Free PMC article.
-
Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication.Nat Cell Biol. 2019 Jan;21(1):9-17. doi: 10.1038/s41556-018-0250-9. Epub 2019 Jan 2. Nat Cell Biol. 2019. PMID: 30602770 Review.
-
Defective HIV-1 particle assembly in AP-3-deficient cells derived from patients with Hermansky-Pudlak syndrome type 2.J Virol. 2012 Oct;86(20):11242-53. doi: 10.1128/JVI.00544-12. Epub 2012 Aug 8. J Virol. 2012. PMID: 22875976 Free PMC article.
References
-
- Swanstrom R., Wills J. W. (1997) in Retroviruses (Coffin J. M., Hughes S. H., Varmus H. E. eds) pp. 263–334, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY - PubMed
-
- Vogt V. M. (1997) in Retroviruses (Coffin J. M., Hughes S. H., Varmus H. E. eds) pp. 27–70, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
-
- Adamson C. S., Freed E. O. (2007) Adv. Pharmacol. 55, 347–387 - PubMed
-
- Bieniasz P. D. (2006) Virology 344, 55–63 - PubMed
-
- Freed E. O. (1998) Virology 251, 1–15 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

