Essential role of maternal UCHL1 and UCHL3 in fertilization and preimplantation embryo development
- PMID: 21678411
- PMCID: PMC4351554
- DOI: 10.1002/jcp.22876
Essential role of maternal UCHL1 and UCHL3 in fertilization and preimplantation embryo development
Abstract
Post-translational protein modification by ubiquitination, a signal for lysosomal or proteasomal proteolysis, can be regulated and reversed by deubiquitinating enzymes (DUBs). This study examined the roles of UCHL1 and UCHL3, two members of ubiquitin C-terminal hydrolase (UCH) family of DUBs, in murine fertilization and preimplantation development. Before fertilization, these proteins were associated with the oocyte cortex (UCHL1) and meiotic spindle (UCHL3). Intracytoplasmic injection of the general UCH-family inhibitor ubiquitin-aldehyde (UBAL) or antibodies against UCHL3 into mature metaphase II oocytes blocked fertilization by reducing sperm penetration of the zona pellucida and incorporation into the ooplasm, suggesting a role for cortical UCHL1 in sperm incorporation. Both UBAL and antibodies against UCHL1 injected at the onset of oocyte maturation (germinal vesicle stage) reduced the fertilizing ability of oocytes. The subfertile Uchl1(gad-/-) mutant mice showed an intriguing pattern of switched UCH localization, with UCHL3 replacing UCHL1 in the oocyte cortex. While fertilization defects were not observed, the embryos from homozygous Uchl1(gad-/-) mutant females failed to undergo morula compaction and did not form blastocysts in vivo, indicating a maternal effect related to UCHL1 deficiency. We conclude that the activity of oocyte UCHs contributes to fertilization and embryogenesis by regulating the physiology of the oocyte and blastomere cortex.
Copyright © 2011 Wiley Periodicals, Inc.
Figures
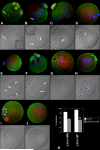
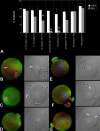
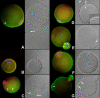
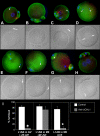
Similar articles
-
Essential role of ubiquitin C-terminal hydrolases UCHL1 and UCHL3 in mammalian oocyte maturation.J Cell Physiol. 2012 May;227(5):2022-9. doi: 10.1002/jcp.22931. J Cell Physiol. 2012. PMID: 21751213 Free PMC article.
-
Ubiquitin C-terminal hydrolase-activity is involved in sperm acrosomal function and anti-polyspermy defense during porcine fertilization.Biol Reprod. 2007 Nov;77(5):780-93. doi: 10.1095/biolreprod.107.061275. Epub 2007 Aug 1. Biol Reprod. 2007. PMID: 17671268
-
Protein deubiquitination during oocyte maturation influences sperm function during fertilisation, antipolyspermy defense and embryo development.Reprod Fertil Dev. 2015 Nov;27(8):1154-67. doi: 10.1071/RD14012. Reprod Fertil Dev. 2015. PMID: 24848520
-
Deubiquitinating enzymes in oocyte maturation, fertilization and preimplantation embryo development.Adv Exp Med Biol. 2014;759:89-110. doi: 10.1007/978-1-4939-0817-2_5. Adv Exp Med Biol. 2014. PMID: 25030761 Review.
-
The new function of two ubiquitin C-terminal hydrolase isozymes as reciprocal modulators of germ cell apoptosis.Exp Anim. 2007 Apr;56(2):71-7. doi: 10.1538/expanim.56.71. Exp Anim. 2007. PMID: 17460351 Review.
Cited by
-
The deubiquitinating enzyme UCHL3 promotes anaplastic thyroid cancer progression and metastasis through Hippo signaling pathway.Cell Death Differ. 2023 May;30(5):1247-1259. doi: 10.1038/s41418-023-01134-z. Epub 2023 Feb 22. Cell Death Differ. 2023. PMID: 36813921 Free PMC article.
-
The Conceivable Functions of Protein Ubiquitination and Deubiquitination in Reproduction.Front Physiol. 2022 Jul 13;13:886261. doi: 10.3389/fphys.2022.886261. eCollection 2022. Front Physiol. 2022. PMID: 35910557 Free PMC article. Review.
-
Post-Translational Modifications in Oocyte Maturation and Embryo Development.Front Cell Dev Biol. 2021 Jun 2;9:645318. doi: 10.3389/fcell.2021.645318. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34150752 Free PMC article. Review.
-
Essential role of ubiquitin C-terminal hydrolases UCHL1 and UCHL3 in mammalian oocyte maturation.J Cell Physiol. 2012 May;227(5):2022-9. doi: 10.1002/jcp.22931. J Cell Physiol. 2012. PMID: 21751213 Free PMC article.
-
A phosphorylation-deubiquitination cascade regulates the BRCA2-RAD51 axis in homologous recombination.Genes Dev. 2016 Dec 1;30(23):2581-2595. doi: 10.1101/gad.289439.116. Epub 2016 Dec 9. Genes Dev. 2016. PMID: 27941124 Free PMC article.
References
-
- Chakravarty S, Bansal P, Sutovsky P, Gupta SK. Role of proteasomal activity in the induction of acrosomal exocytosis in human spermatozoa. Reprod Biomed Online. 2008;16(3):391–400. - PubMed
-
- Ellederova Z, Halada P, Man P, Kubelka M, Motlik J, Kovarova H. Protein patterns of pig oocytes during in vitro maturation. Biol Reprod. 2004;71(5):1533–1539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

