Galectin-7 modulates the length of the primary cilia and wound repair in polarized kidney epithelial cells
- PMID: 21677144
- PMCID: PMC3174547
- DOI: 10.1152/ajprenal.00134.2011
Galectin-7 modulates the length of the primary cilia and wound repair in polarized kidney epithelial cells
Abstract
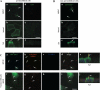
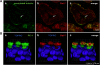
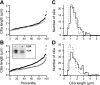
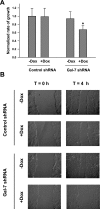
Galectins (Gal) are β-galactoside-binding proteins that function in epithelial development and homeostasis. An overlapping role for Gal-3 and Gal-7 in wound repair was reported in stratified epithelia. Although Gal-7 was thought absent in simple epithelia, it was reported in a proteomic analysis of cilia isolated from cultured human airway, and we recently identified Gal-7 transcripts in Madin-Darby canine kidney (MDCK) cells (Poland PA, Rondanino C, Kinlough CL, Heimburg-Molinaro J, Arthur CM, Stowell SR, Smith DF, Hughey RP. J Biol Chem 286: 6780-6790, 2011). We now report that Gal-7 is localized exclusively on the primary cilium of MDCK, LLC-PK(1) (pig kidney), and mpkCCD(c14) (mouse kidney) cells as well as on cilia in the rat renal proximal tubule. Gal-7 is also present on most cilia of multiciliated cells in human airway epithelia primary cultures. Interestingly, exogenous glutathione S-transferase (GST)-Gal-7 bound the MDCK apical plasma membrane as well as the cilium, while the lectin Ulex europeaus agglutinin, with glycan preferences similar to Gal-7, bound the basolateral plasma membrane as well as the cilium. In pull-down assays, β1-integrin isolated from either the basolateral or apical/cilia membranes of MDCK cells was similarly bound by GST-Gal-7. Selective localization of Gal-7 to cilia despite the presence of binding sites on all cell surfaces suggests that intracellular Gal-7 is specifically delivered to cilia rather than simply binding to surface glycoconjugates after generalized secretion. Moreover, depletion of Gal-7 using tetracycline-induced short-hairpin RNA in mpkCCD(c14) cells significantly reduced cilia length and slowed wound healing in a scratch assay. We conclude that Gal-7 is selectively targeted to cilia and plays a key role in surface stabilization of glycoconjugates responsible for integrating cilia function with epithelial repair.
Figures


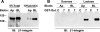
Similar articles
-
Identification and characterization of endogenous galectins expressed in Madin Darby canine kidney cells.J Biol Chem. 2011 Feb 25;286(8):6780-90. doi: 10.1074/jbc.M110.179002. Epub 2010 Dec 2. J Biol Chem. 2011. PMID: 21127048 Free PMC article.
-
Galectin-8 induces partial epithelial-mesenchymal transition with invasive tumorigenic capabilities involving a FAK/EGFR/proteasome pathway in Madin-Darby canine kidney cells.Mol Biol Cell. 2018 Mar 1;29(5):557-574. doi: 10.1091/mbc.E16-05-0301. Epub 2018 Jan 3. Mol Biol Cell. 2018. PMID: 29298841 Free PMC article.
-
Galectin-3 modulates the polarized surface delivery of β1-integrin in epithelial cells.J Cell Sci. 2018 Jun 11;131(11):jcs213199. doi: 10.1242/jcs.213199. J Cell Sci. 2018. PMID: 29748377
-
The renal cell primary cilium functions as a flow sensor.Curr Opin Nephrol Hypertens. 2003 Sep;12(5):517-20. doi: 10.1097/00041552-200309000-00006. Curr Opin Nephrol Hypertens. 2003. PMID: 12920399 Review.
-
Recycling of galectin-3 in epithelial cells.Eur J Cell Biol. 2015 Jul-Sep;94(7-9):309-15. doi: 10.1016/j.ejcb.2015.05.004. Epub 2015 Jun 1. Eur J Cell Biol. 2015. PMID: 26059399 Review.
Cited by
-
Prevalence, Risk Factors, Pathophysiology, Potential Biomarkers and Management of Feline Idiopathic Cystitis: An Update Review.Front Vet Sci. 2022 Jun 21;9:900847. doi: 10.3389/fvets.2022.900847. eCollection 2022. Front Vet Sci. 2022. PMID: 35812890 Free PMC article. Review.
-
Examination of galectin localization using confocal microscopy.Methods Mol Biol. 2015;1207:343-54. doi: 10.1007/978-1-4939-1396-1_23. Methods Mol Biol. 2015. PMID: 25253152 Free PMC article.
-
Galectin-7 promotes the invasiveness of human oral squamous cell carcinoma cells via activation of ERK and JNK signaling.Oncol Lett. 2017 Mar;13(3):1919-1924. doi: 10.3892/ol.2017.5649. Epub 2017 Jan 25. Oncol Lett. 2017. PMID: 28454344 Free PMC article.
-
A Mutation in the Carbohydrate Recognition Domain Drives a Phenotypic Switch in the Role of Galectin-7 in Prostate Cancer.PLoS One. 2015 Jul 13;10(7):e0131307. doi: 10.1371/journal.pone.0131307. eCollection 2015. PLoS One. 2015. PMID: 26168167 Free PMC article.
-
Aurora kinase A activates the vacuolar H+-ATPase (V-ATPase) in kidney carcinoma cells.Am J Physiol Renal Physiol. 2016 Jun 1;310(11):F1216-28. doi: 10.1152/ajprenal.00061.2016. Epub 2016 Feb 24. Am J Physiol Renal Physiol. 2016. PMID: 26911844 Free PMC article.
References
-
- Bacallao R, Stelzer EH. Preservation of biological specimens for observation in a confocal fluorescence microscope and operational principles of confocal fluorescence microscopy. Methods Cell Biol 31: 437–452, 1989 - PubMed
-
- Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999 - PubMed
-
- Brown D, Lydon J, McLaughlin M, Stuart-Tilley A, Tyszkowski R, Alper S. Antigen retrieval in cryostat tissue sections and cultured cells by treatment with sodium dodecyl sulfate (SDS). Histochem Cell Biol 105: 261–267, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK084184/DK/NIDDK NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- DK54787/DK/NIDDK NIH HHS/United States
- P30 DK072506/DK/NIDDK NIH HHS/United States
- R01 DK075048-04/DK/NIDDK NIH HHS/United States
- R01 DK075048/DK/NIDDK NIH HHS/United States
- DK054407/DK/NIDDK NIH HHS/United States
- K01 DK078734/DK/NIDDK NIH HHS/United States
- DK075048/DK/NIDDK NIH HHS/United States
- R01 HL112863/HL/NHLBI NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- P30 DK079307/DK/NIDDK NIH HHS/United States
- K08 HL087932/HL/NHLBI NIH HHS/United States
- R01 DK075048-05/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

