Biosynthesis of promatrix metalloproteinase-9/chondroitin sulphate proteoglycan heteromer involves a Rottlerin-sensitive pathway
- PMID: 21673806
- PMCID: PMC3105995
- DOI: 10.1371/journal.pone.0020616
Biosynthesis of promatrix metalloproteinase-9/chondroitin sulphate proteoglycan heteromer involves a Rottlerin-sensitive pathway
Abstract
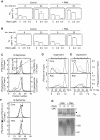
Background: Previously we have shown that a fraction of the matrix metalloproteinase-9 (MMP-9) synthesized by the macrophage cell line THP-1 was bound to a chondroitin sulphate proteoglycan (CSPG) core protein as a reduction sensitive heteromer. Several biochemical properties of the enzyme were changed when it was bound to the CSPG.
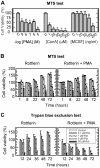
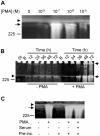
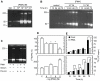
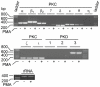
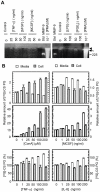
Methodology/principal findings: By use of affinity chromatography, zymography, and radioactive labelling, various macrophage stimulators were tested for their effect on the synthesis of the proMMP-9/CSPG heteromer and its components by THP-1 cells. Of the stimulators, only PMA largely increased the biosynthesis of the heteromer. As PMA is an activator of PKC, we determined which PKC isoenzymes were expressed by performing RT-PCR and Western Blotting. Subsequently specific inhibitors were used to investigate their involvement in the biosynthesis of the heteromer. Of the inhibitors, only Rottlerin repressed the biosynthesis of proMMP-9/CSPG and its two components. Much lower concentrations of Rottlerin were needed to reduce the amount of CSPG than what was needed to repress the synthesis of the heteromer and MMP-9. Furthermore, Rottlerin caused a minor reduction in the activation of the PKC isoenzymes δ, ε, θ and υ (PKD3) in both control and PMA exposed cells.
Conclusions/significance: The biosynthesis of the proMMP-9/CSPG heteromer and proMMP-9 in THP-1 cells involves a Rottlerin-sensitive pathway that is different from the Rottlerin sensitive pathway involved in the CSPG biosynthesis. MMP-9 and CSPGs are known to be involved in various physiological and pathological processes. Formation of complexes may influence both the specificity and localization of the enzyme. Therefore, knowledge about biosynthetic pathways and factors involved in the formation of the MMP-9/CSPG heteromer may contribute to insight in the heteromers biological function as well as pointing to future targets for therapeutic agents.
Conflict of interest statement
Figures


Similar articles
-
In vitro reconstitution of complexes between pro-matrix metalloproteinase-9 and the proteoglycans serglycin and versican.FEBS J. 2013 Jun;280(12):2870-87. doi: 10.1111/febs.12291. Epub 2013 May 9. FEBS J. 2013. PMID: 23601700
-
Calcium-induced activation and truncation of promatrix metalloproteinase-9 linked to the core protein of chondroitin sulfate proteoglycans.Eur J Biochem. 2003 Oct;270(19):3996-4007. doi: 10.1046/j.1432-1033.2003.03788.x. Eur J Biochem. 2003. PMID: 14511382
-
Interaction of pro-matrix metalloproteinase-9/proteoglycan heteromer with gelatin and collagen.J Biol Chem. 2008 May 16;283(20):13652-65. doi: 10.1074/jbc.M709140200. Epub 2008 Mar 20. J Biol Chem. 2008. PMID: 18359769
-
Rottlerin, a natural polyphenol compound, inhibits upregulation of matrix metalloproteinase-9 and brain astrocytic migration by reducing PKC-δ-dependent ROS signal.J Neuroinflammation. 2020 Jun 6;17(1):177. doi: 10.1186/s12974-020-01859-5. J Neuroinflammation. 2020. PMID: 32505192 Free PMC article.
-
Rottlerin and cancer: novel evidence and mechanisms.ScientificWorldJournal. 2012;2012:350826. doi: 10.1100/2012/350826. Epub 2012 Jan 4. ScientificWorldJournal. 2012. PMID: 22272173 Free PMC article. Review.
Cited by
-
The selectivity of galardin and an azasugar-based hydroxamate compound for human matrix metalloproteases and bacterial metalloproteases.PLoS One. 2018 Aug 3;13(8):e0200237. doi: 10.1371/journal.pone.0200237. eCollection 2018. PLoS One. 2018. PMID: 30075004 Free PMC article.
-
Inhibition of bacterial and human zinc-metalloproteases by bisphosphonate- and catechol-containing compounds.J Enzyme Inhib Med Chem. 2021 Dec;36(1):819-830. doi: 10.1080/14756366.2021.1901088. J Enzyme Inhib Med Chem. 2021. PMID: 33757387 Free PMC article.
-
Evaluation of protein kinase D auto-phosphorylation as biomarker for NLRP3 inflammasome activation.PLoS One. 2021 Nov 12;16(11):e0248668. doi: 10.1371/journal.pone.0248668. eCollection 2021. PLoS One. 2021. PMID: 34767572 Free PMC article.
-
Molecular Interactions Stabilizing the Promatrix Metalloprotease-9·Serglycin Heteromer.Int J Mol Sci. 2020 Jun 12;21(12):4205. doi: 10.3390/ijms21124205. Int J Mol Sci. 2020. PMID: 32545641 Free PMC article.
References
-
- Hascall VC, Calabro A, Midura RJ, Yanagishita M. Isolation and characterization of proteoglycans. Methods Enzymol. 1994;230:390–417. - PubMed
-
- Kjellen L, Lindahl U. Proteoglycans: structures and interactions [published erratum appears in Annu Rev Biochem 1992;61:following viii]. Annu Rev Biochem. 1991;60:443–475. - PubMed
-
- Edwards IJ, Xu H, Obunike JC, Goldberg IJ, Wagner WD. Differentiated macrophages synthesize a heparan sulphate proteoglycan and an oversulphated chondroitin sulphate proteoglycan that bind lipoprotein lipase. Arterioscler Thromb Vasc Biol. 1995;15:400–409. - PubMed
-
- Kolset SO, Gallagher JT. Proteoglycans in haemopoietic cells. Biochim Biophys Acta. 1990;1032:191–211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

