Safety and immunogenicity of early measles vaccination in children born to HIV-infected mothers in the United States: results of Pediatric AIDS Clinical Trials Group (PACTG) protocol 225
- PMID: 21666159
- PMCID: PMC3143449
- DOI: 10.1093/infdis/jir089
Safety and immunogenicity of early measles vaccination in children born to HIV-infected mothers in the United States: results of Pediatric AIDS Clinical Trials Group (PACTG) protocol 225
Abstract
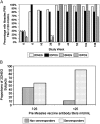
BACKGROUND.: ACTG (Pediatric AIDS Clinical Trials Group) 225, a multicenter, randomized, open-label trial in the United States evaluated reactogenicity and immunogenicity of 2 vaccination regimens: monovalent measles vaccine (Attenuvax) at 6 months of age and measles, mumps, and rubella, live attenuated (MMRII) vaccine at 12 months of age (2D), or only MMRII at 12 months of age (1D) in human immunodeficiency virus-infected (HIV-infected) (POS) and uninfected (NEG) children in the pre-highly active antiretroviral therapy (pre-HAART) period.
Methods: Plaque-reduction neutralization (PRN) of measles-neutralizing antibody titers were evaluated at study weeks 0, 6, 26, 32, 52, and 130 (∼3 years of age).
Results: The 110 subjects included: 65 2DNEG; 30 1DNEG; 7 2DPOS and 8 1DPOS. Vaccinations (n=175) were associated with no adverse experiences >Grade 2 except for Grade 3 fever (n=2, 1 1DPOS and 1 1DNEG). Six weeks after Attenuvax, all 2DPOS subjects (7/7) seroresponded (PRN titers ≥120 mIU/mL) with median titers significantly exceeding 2DNEG titers (2115 vs 628 mIU/mL, respectively; P=.023). At ∼3 years of age, 67% 1DPOS (4/6) and 83% 2DPOS (4/5) subjects maintained titers ≥120 mIU/mL. Prevaccination titers ≥25 mIU/mL among 2DNEG subjects correlated inversely with the likelihood of achieving titers ≥120 mIU/mL (56% vs 90%; P=.004).
Conclusions: Among HIV-infected children pre-HAART, Attenuvax at 6 months was well tolerated and immunogenic. These data support the current World Health Organization (WHO) recommendation to administer a first dose of measles vaccine at 6 months of age to HIV-infected children.
© The Author 2011. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved.
Figures


Similar articles
-
Immunogenicity, immunologic memory, and safety following measles revaccination in HIV-infected children receiving highly active antiretroviral therapy.J Infect Dis. 2012 Aug 15;206(4):512-22. doi: 10.1093/infdis/jis386. Epub 2012 Jun 12. J Infect Dis. 2012. PMID: 22693229 Free PMC article. Clinical Trial.
-
Immunogenicity and Safety of an Early Measles Vaccination Schedule at 6 and 12 Months of Age in Human Immunodeficiency Virus (HIV)-Unexposed and HIV-Exposed, Uninfected South African Children.J Infect Dis. 2019 Sep 26;220(9):1529-1538. doi: 10.1093/infdis/jiz348. J Infect Dis. 2019. PMID: 31282539
-
Immunogenicity and efficacy of one dose measles-mumps-rubella (MMR) vaccine at twelve months of age as compared to monovalent measles vaccination at nine months followed by MMR revaccination at fifteen months of age.Vaccine. 2001 Aug 14;19(31):4473-8. doi: 10.1016/s0264-410x(01)00207-9. Vaccine. 2001. PMID: 11483273 Clinical Trial.
-
Live attenuated measles, mumps, rubella, and varicella zoster virus vaccine (Priorix-Tetra).Paediatr Drugs. 2008;10(5):337-47. doi: 10.2165/00148581-200810050-00007. Paediatr Drugs. 2008. PMID: 18754700 Review.
-
Measles vaccination in HIV-infected children: systematic review and meta-analysis of safety and immunogenicity.J Infect Dis. 2011 Jul;204 Suppl 1:S164-78. doi: 10.1093/infdis/jir071. J Infect Dis. 2011. PMID: 21666158 Review.
Cited by
-
Subacute sclerosing panencephalitis in a child with human immunodeficiency virus (HIV) infection on antiretroviral therapy.Ann Indian Acad Neurol. 2015 Jan-Mar;18(1):96-8. doi: 10.4103/0972-2327.144299. Ann Indian Acad Neurol. 2015. PMID: 25745323 Free PMC article.
-
Safety and Immunogenicity of Measles Vaccination in HIV-Infected and HIV-Exposed Uninfected Children: A Systematic Review and Meta-Analysis.EClinicalMedicine. 2018 Jul 2;1:28-42. doi: 10.1016/j.eclinm.2018.06.002. eCollection 2018 Jul. EClinicalMedicine. 2018. PMID: 31193646 Free PMC article.
-
[An investigation of vaccination in children with human immunodeficiency virus infection].Zhongguo Dang Dai Er Ke Za Zhi. 2019 Mar;21(3):199-202. doi: 10.7499/j.issn.1008-8830.2019.03.002. Zhongguo Dang Dai Er Ke Za Zhi. 2019. PMID: 30907339 Free PMC article. Chinese.
-
Seroprevalence of transplacentally acquired measles antibodies in HIV-exposed versus HIV-unexposed infants at six months of age.Indian J Med Res. 2017 Apr;145(4):536-542. doi: 10.4103/ijmr.IJMR_44_16. Indian J Med Res. 2017. PMID: 28862187 Free PMC article.
-
Subacute Sclerosing Panencephalitis in a Child Suffering from Human Immunodeficiency Virus on "Highly Active Antiretroviral Therapy" - Can This be Another Instance of Immune Reconstitution Inflammatory Syndrome?Cureus. 2017 Jun 13;9(6):e1346. doi: 10.7759/cureus.1346. Cureus. 2017. PMID: 28713662 Free PMC article.
References
-
- Enders JF, Carthy Mc, Mitus A, Cheatham WJ. Isolation of measles virus at autopsy in cases of giant-cell pneumonia without rash. N Engl J Med. 1959;261:875–81. - PubMed
-
- Centers for Disease Control. Measles in HIV-1 infected children, United States. MMWR. 1988;37:183–6. - PubMed
-
- McLaughlin M, Thomas P, Onorato I, et al. Live virus vaccines in human immunodeficiency virus-infected children: a retrospective survey. Pediatrics. 1988;82:229–33. - PubMed
-
- Centers for Disease Control. General recommendation on immunization—Recommendation of the Advisory Committee on Immunization Practices (ACIP) MMWR. 1994;43:22–5.
-
- Angel JB, Walpita P, Lerch RA, et al. Vaccine-associated measles pneumonitis in an adult with AIDS. Ann Intern Med. 1998;129:104–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

