Nicotine reduces L-DOPA-induced dyskinesias by acting at beta2* nicotinic receptors
- PMID: 21665941
- PMCID: PMC3164339
- DOI: 10.1124/jpet.111.182949
Nicotine reduces L-DOPA-induced dyskinesias by acting at beta2* nicotinic receptors
Abstract
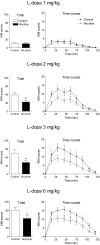
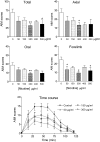
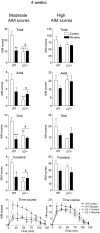
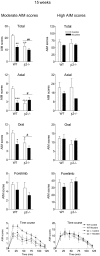
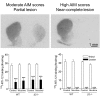
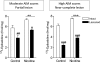
L-DOPA-induced dyskinesias or abnormal involuntary movements (AIMs) are a debilitating adverse complication associated with prolonged L-DOPA administration for Parkinson's disease. Few treatments are currently available for dyskinesias. Our recent data showed that nicotine reduced L-DOPA-induced AIMs in parkinsonian animal models. An important question is the nicotinic acetylcholine receptor (nAChR) subtypes through which nicotine exerts this beneficial effect, because such knowledge would allow for the development of drugs that target the relevant receptor population(s). To address this, we used β2 nAChR subunit knockout [β2(-/-)] mice because β2-containing nAChRs are key regulators of nigrostriatal dopaminergic function. All of the mice were lesioned by intracranial injection of 6-hydroxydopamine into the right medial forebrain bundle. Lesioning resulted in a similar degree of nigrostriatal damage and parkinsonism in β2(-/-) and wild-type mice. All of the mice then were injected with L-DOPA (3 mg/kg) plus benserazide (15 mg/kg) once daily for 4 weeks until AIMs were fully developed. L-DOPA-induced AIMs were approximately 40% less in the β2(-/-) mice compared with the wild-type mice. It is interesting to note that nicotine (300 μg/ml in drinking water) reduced L-DOPA-induced AIMs by 40% in wild-type mice but had no effect in β2(-/-) mice with partial nigrostriatal damage. The nicotine-mediated decline in AIMs was much less pronounced in wild-type mice with near-complete degeneration, suggesting that presynaptic nAChRs on dopaminergic terminals have a major influence. These data demonstrate an essential role for β2* nAChRs in the antidyskinetic effect of nicotine and suggest that drugs targeting these subtypes may be useful for the management of L-DOPA-induced dyskinesias in Parkinson's disease.
Figures
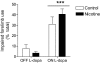

Similar articles
-
Role for α6 nicotinic receptors in l-dopa-induced dyskinesias in parkinsonian mice.Neuropharmacology. 2012 Sep;63(3):450-9. doi: 10.1016/j.neuropharm.2012.04.029. Epub 2012 May 3. Neuropharmacology. 2012. PMID: 22579614 Free PMC article.
-
Nicotinic receptor-mediated reduction in L-DOPA-induced dyskinesias may occur via desensitization.J Pharmacol Exp Ther. 2010 Jun;333(3):929-38. doi: 10.1124/jpet.109.162396. Epub 2010 Mar 3. J Pharmacol Exp Ther. 2010. PMID: 20200117 Free PMC article.
-
Multiple CNS nicotinic receptors mediate L-dopa-induced dyskinesias: studies with parkinsonian nicotinic receptor knockout mice.Biochem Pharmacol. 2013 Oct 15;86(8):1153-62. doi: 10.1016/j.bcp.2013.06.027. Epub 2013 Jul 4. Biochem Pharmacol. 2013. PMID: 23831952 Free PMC article.
-
Role for the nicotinic cholinergic system in movement disorders; therapeutic implications.Pharmacol Ther. 2014 Oct;144(1):50-9. doi: 10.1016/j.pharmthera.2014.05.004. Epub 2014 May 14. Pharmacol Ther. 2014. PMID: 24836728 Free PMC article. Review.
-
Multiple roles for nicotine in Parkinson's disease.Biochem Pharmacol. 2009 Oct 1;78(7):677-85. doi: 10.1016/j.bcp.2009.05.003. Epub 2009 May 9. Biochem Pharmacol. 2009. PMID: 19433069 Free PMC article. Review.
Cited by
-
Nicotine as a potential neuroprotective agent for Parkinson's disease.Mov Disord. 2012 Jul;27(8):947-57. doi: 10.1002/mds.25028. Epub 2012 Jun 12. Mov Disord. 2012. PMID: 22693036 Free PMC article. Review.
-
Genetic Knockdown of mGluR5 in Striatal D1R-Containing Neurons Attenuates L-DOPA-Induced Dyskinesia in Aphakia Mice.Mol Neurobiol. 2019 Jun;56(6):4037-4050. doi: 10.1007/s12035-018-1356-6. Epub 2018 Sep 27. Mol Neurobiol. 2019. PMID: 30259400
-
Nicotinic receptor agonists reduce L-DOPA-induced dyskinesias in a monkey model of Parkinson's disease.J Pharmacol Exp Ther. 2013 Oct;347(1):225-34. doi: 10.1124/jpet.113.207639. Epub 2013 Jul 31. J Pharmacol Exp Ther. 2013. PMID: 23902940 Free PMC article.
-
Analysis of gait in rats with olivocerebellar lesions and ability of the nicotinic acetylcholine receptor agonist varenicline to attenuate impairments.Behav Brain Res. 2015 Sep 15;291:342-350. doi: 10.1016/j.bbr.2015.05.056. Epub 2015 Jun 3. Behav Brain Res. 2015. PMID: 26049061 Free PMC article.
-
Neuronal nicotinic receptor agonists improve gait and balance in olivocerebellar ataxia.Neuropharmacology. 2013 Oct;73:75-86. doi: 10.1016/j.neuropharm.2013.05.016. Epub 2013 May 24. Neuropharmacology. 2013. PMID: 23711550 Free PMC article.
References
-
- Abin-Carriquiry JA, Urbanavicius J, Scorza C, Rebolledo-Fuentes M, Wonnacott S, Cassels BK, Dajas F. (2010) Increase in locomotor activity after acute administration of the nicotinic receptor agonist 3-bromocytisine in rats. Eur J Pharmacol 634:89–94 - PubMed
-
- Artymyshyn R, Smith A, Wolfe BB. (1990) The use of 3H standards in 125I autoradiography. J Neurosci Methods 32:185–192 - PubMed
-
- Barik J, Wonnacott S. (2009) Molecular and cellular mechanisms of action of nicotine in the CNS. Handb Exp Pharmacol 192:173–207 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
