Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function
- PMID: 21664576
- PMCID: PMC3121104
- DOI: 10.1016/j.devcel.2011.04.018
Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function
Abstract
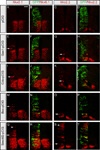
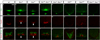
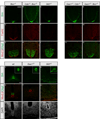
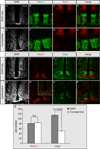
Secreted Hedgehog (HH) ligands signal through the canonical receptor Patched (PTCH1). However, recent studies implicate three additional HH-binding, cell-surface proteins, GAS1, CDO, and BOC, as putative coreceptors for HH ligands. A central question is to what degree these coreceptors function similarly and what their collective requirement in HH signal transduction is. Here we provide evidence that GAS1, CDO, and BOC play overlapping and essential roles during HH-mediated ventral neural patterning of the mammalian neural tube. Specifically, we demonstrate two important roles for these molecules: an early role in cell fate specification of multiple neural progenitors and a later role in motor neuron progenitor maintenance. Most strikingly, genetic loss-of-function experiments indicate an obligatory requirement for GAS1, CDO, and BOC in HH pathway activity in multiple tissues.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation.Dev Cell. 2011 Jun 14;20(6):788-801. doi: 10.1016/j.devcel.2011.04.017. Dev Cell. 2011. PMID: 21664577 Free PMC article.
-
The hedgehog co-receptor BOC differentially regulates SHH signaling during craniofacial development.Development. 2020 Dec 14;147(23):dev189076. doi: 10.1242/dev.189076. Development. 2020. PMID: 33060130 Free PMC article.
-
CDON contributes to Hedgehog-dependent patterning and growth of the developing limb.Dev Biol. 2023 Jan;493:1-11. doi: 10.1016/j.ydbio.2022.09.011. Epub 2022 Oct 17. Dev Biol. 2023. PMID: 36265686
-
The role of Gas1 in embryonic development and its implications for human disease.Cell Cycle. 2007 Nov 1;6(21):2650-5. doi: 10.4161/cc.6.21.4877. Epub 2007 Aug 13. Cell Cycle. 2007. PMID: 17726382 Review.
-
Cdon and Boc: Two transmembrane proteins implicated in cell-cell communication.Int J Biochem Cell Biol. 2012 May;44(5):698-702. doi: 10.1016/j.biocel.2012.01.019. Epub 2012 Feb 3. Int J Biochem Cell Biol. 2012. PMID: 22326621 Review.
Cited by
-
Cdo Is Required for Efficient Motor Neuron Generation of Embryonic Stem Cells.Int J Stem Cells. 2020 Nov 30;13(3):342-352. doi: 10.15283/ijsc20037. Int J Stem Cells. 2020. PMID: 32840224 Free PMC article.
-
Ihog proteins contribute to integrin-mediated focal adhesions.Sci China Life Sci. 2023 Feb;66(2):366-375. doi: 10.1007/s11427-022-2154-1. Epub 2022 Sep 8. Sci China Life Sci. 2023. PMID: 36103028
-
Overweight in mice and enhanced adipogenesis in vitro are associated with lack of the hedgehog coreceptor boc.Diabetes. 2015 Jun;64(6):2092-103. doi: 10.2337/db14-1017. Epub 2015 Jan 9. Diabetes. 2015. PMID: 25576054 Free PMC article.
-
Menin directly represses Gli1 expression independent of canonical Hedgehog signaling.Mol Cancer Res. 2013 Oct;11(10):1215-22. doi: 10.1158/1541-7786.MCR-13-0170. Epub 2013 Aug 8. Mol Cancer Res. 2013. PMID: 23928057 Free PMC article.
-
Arx together with FoxA2, regulates Shh floor plate expression.Dev Biol. 2014 Sep 1;393(1):137-48. doi: 10.1016/j.ydbio.2014.06.012. Epub 2014 Jun 23. Dev Biol. 2014. PMID: 24968361 Free PMC article.
References
-
- Airaksinen MS, Holm L, Hatinen T. Evolution of the GDNF family ligands and receptors. Brain Behav Evol. 2006;68:181–190. - PubMed
-
- Briscoe J, Ericson J. Specification of neuronal fates in the ventral neural tube. Curr Opin Neurobiol. 2001;11:43–49. - PubMed
-
- Cabrera JR, Sanchez-Pulido L, Rojas AM, Valencia A, Manes S, Naranjo JR, Mellstrom B. Gas1 is related to the glial cell-derived neurotrophic factor family receptors alpha and regulates Ret signaling. J Biol Chem. 2006;281:14330–14339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

