The road less traveled: new views of steroid receptor action from the path of dose-response curves
- PMID: 21664235
- PMCID: PMC3184374
- DOI: 10.1016/j.mce.2011.05.030
The road less traveled: new views of steroid receptor action from the path of dose-response curves
Abstract
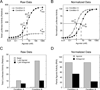
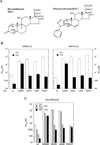
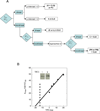
Conventional studies of steroid hormone action proceed via quantitation of the maximal activity for gene induction at saturating concentrations of agonist steroid (i.e., A(max)). Less frequently analyzed parameters of receptor-mediated gene expression are EC(50) and PAA. The EC(50) is the concentration of steroid required for half-maximal agonist activity and is readily determined from the dose-response curve. The PAA is the partial agonist activity of an antagonist steroid, expressed as percent of A(max) under the same conditions. Recent results demonstrate that new and otherwise inaccessible mechanistic information is obtained when the EC(50) and/or PAA are examined in addition to the A(max). Specifically, A(max), EC(50), and PAA can be independently regulated, which suggests that novel pathways and factors may preferentially modify the EC(50) and/or PAA with little effect on A(max). Other approaches indicate that the activity of receptor-bound factors can be altered without changing the binding of factors to receptor. Finally, a new theoretical model of steroid hormone action not only permits a mechanistically based definition of factor activity but also allows the positioning of when a factor acts, as opposed to binds, relative to a kinetically defined step. These advances illustrate some of the benefits of expanding the mechanistic studies of steroid hormone action to routinely include EC(50) and PAA.
Published by Elsevier Ireland Ltd.
Figures



Similar articles
-
Separate regions of glucocorticoid receptor, coactivator TIF2, and comodulator STAMP modify different parameters of glucocorticoid-mediated gene induction.Mol Cell Endocrinol. 2012 May 15;355(1):121-34. doi: 10.1016/j.mce.2012.02.001. Epub 2012 Feb 11. Mol Cell Endocrinol. 2012. PMID: 22342989 Free PMC article.
-
The importance of being varied in steroid receptor transactivation.Trends Pharmacol Sci. 2003 May;24(5):253-9. doi: 10.1016/S0165-6147(03)00101-9. Trends Pharmacol Sci. 2003. PMID: 12767725 Review.
-
New antiprogestins with partial agonist activity: potential selective progesterone receptor modulators (SPRMs) and probes for receptor- and coregulator-induced changes in progesterone receptor induction properties.Mol Endocrinol. 2001 Feb;15(2):255-70. doi: 10.1210/mend.15.2.0596. Mol Endocrinol. 2001. PMID: 11158332
-
How much is enough? Modulation of dose-response curve for steroid receptor-regulated gene expression by changing concentrations of transcription factor.Curr Top Med Chem. 2006;6(3):271-85. doi: 10.2174/156802606776173465. Curr Top Med Chem. 2006. PMID: 16515481 Review.
-
Competitive Agonists and Antagonists of Steroid Nuclear Receptors: Evolution of the Concept or Its Reversal.Biochemistry (Mosc). 2015 Oct;80(10):1227-34. doi: 10.1134/S000629791510003X. Biochemistry (Mosc). 2015. PMID: 26567566 Review.
Cited by
-
Eukaryotic transcriptional dynamics: from single molecules to cell populations.Nat Rev Genet. 2013 Aug;14(8):572-84. doi: 10.1038/nrg3484. Epub 2013 Jul 9. Nat Rev Genet. 2013. PMID: 23835438 Free PMC article. Review.
-
Glucocorticoid-independent modulation of GR activity: Implications for immunotherapy.Pharmacol Ther. 2016 Sep;165:93-113. doi: 10.1016/j.pharmthera.2016.06.002. Epub 2016 Jun 8. Pharmacol Ther. 2016. PMID: 27288728 Free PMC article. Review.
-
An Approach to Greater Specificity for Glucocorticoids.Front Endocrinol (Lausanne). 2018 Mar 13;9:76. doi: 10.3389/fendo.2018.00076. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29593646 Free PMC article. Review.
-
Identification of location and kinetically defined mechanism of cofactors and reporter genes in the cascade of steroid-regulated transactivation.J Biol Chem. 2012 Nov 30;287(49):40982-95. doi: 10.1074/jbc.M112.414805. Epub 2012 Oct 10. J Biol Chem. 2012. PMID: 23055525 Free PMC article.
-
Minireview: dynamic structures of nuclear hormone receptors: new promises and challenges.Mol Endocrinol. 2014 Feb;28(2):173-82. doi: 10.1210/me.2013-1334. Epub 2013 Nov 27. Mol Endocrinol. 2014. PMID: 24284822 Free PMC article. Review.
References
-
- Biggadike K, Bledsoe RK, Coe DM, Cooper TW, House D, Iannone MA, Macdonald SJ, Madauss KP, McLay IM, Shipley TJ, Taylor SJ, Tran TB, Uings IJ, Weller V, Williams SP. Design and x-ray crystal structures of high-potency nonsteroidal glucocorticoid agonists exploiting a novel binding site on the receptor. Proc Natl Acad Sci U S A. 2009;106:18114–18119. - PMC - PubMed
-
- Bledsoe RK, Montana VG, Stanley TB, Delves CJ, Apolito CJ, McKee DD, Consler TG, Parks DJ, Stewart EL, Willson TM, Lambert MH, Moore JT, Pearce KH, Xu HE. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell. 2002;110:93–105. - PubMed
-
- Cho S, Kagan BL, Blackford JA, Jr, Szapary D, Simons SS., Jr Glucocorticoid receptor ligand binding domain is sufficient for the modulation of glucocorticoid induction properties by homologous receptors, coactivator transcription intermediary factor 2, and Ubc9. Mol. Endo. 2005;19:290–311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

