Protein kinase R as mediator of the effects of interferon (IFN) gamma and tumor necrosis factor (TNF) alpha on normal and dysplastic hematopoiesis
- PMID: 21659535
- PMCID: PMC3149343
- DOI: 10.1074/jbc.M111.238501
Protein kinase R as mediator of the effects of interferon (IFN) gamma and tumor necrosis factor (TNF) alpha on normal and dysplastic hematopoiesis
Abstract
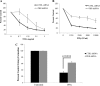
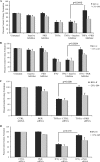
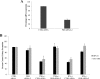
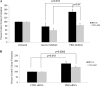
IFNγ and TNFα are potent inhibitors of hematopoiesis and have been implicated in the pathophysiology of bone marrow failure and myelodysplastic syndromes (MDS). We examined the role of protein kinase R (PKR) in the generation of the inhibitory effects of these myelosuppressive cytokines on hematopoiesis. Our data demonstrate that PKR is rapidly phosphorylated/activated in response to engagement of IFNγ or TNFα receptors in normal human hematopoietic progenitors. Such engagement of PKR is important for the suppressive effects of these cytokines on normal hematopoiesis. Pharmacological targeting of PKR using a specific inhibitor or siRNA-mediated PKR knockdown results in partial reversal of the suppressive effects of IFNγ and TNFα on normal human CD34+-derived myeloid (colony-forming unit-granulocyte-monocytic) and erythroid (burst-forming unit-erythroid) progenitors. Importantly, inhibition of PKR activity or expression increases hematopoietic colony formation from human MDS progenitors, suggesting that drugs that target PKR may provide a novel approach for the treatment of MDS and marrow failure syndromes. Altogether, our data establish that beyond its key role in the induction of IFN-antiviral responses, PKR plays important roles in signaling for IFNγ and other myelosuppressive cytokine receptors as a common mediator of signals for hematopoietic suppression.
Figures






Similar articles
-
Role of the p38 mitogen-activated protein kinase pathway in cytokine-mediated hematopoietic suppression in myelodysplastic syndromes.Cancer Res. 2005 Oct 1;65(19):9029-37. doi: 10.1158/0008-5472.CAN-04-4555. Cancer Res. 2005. PMID: 16204077
-
Activation of the p38 mitogen-activated protein kinase mediates the suppressive effects of type I interferons and transforming growth factor-beta on normal hematopoiesis.J Biol Chem. 2002 Mar 8;277(10):7726-35. doi: 10.1074/jbc.M106640200. Epub 2001 Dec 31. J Biol Chem. 2002. PMID: 11773065
-
Interferon-gamma constitutively expressed in the stromal microenvironment of human marrow cultures mediates potent hematopoietic inhibition.Blood. 1996 May 15;87(10):4149-57. Blood. 1996. PMID: 8639773
-
TNF-alpha, the great imitator: role of p55 and p75 TNF receptors in hematopoiesis.Stem Cells. 1994;12 Suppl 1:111-26; discussion 126-8. Stem Cells. 1994. PMID: 7535144 Review.
-
Hematopoietic progenitors and synergism of interferon-gamma and stem cell factor.Leuk Lymphoma. 1994 Jul;14(3-4):203-11. doi: 10.3109/10428199409049670. Leuk Lymphoma. 1994. PMID: 7524887 Review.
Cited by
-
Roles of E4orf6 and VA I RNA in adenovirus-mediated stimulation of human parvovirus B19 DNA replication and structural gene expression.J Virol. 2012 May;86(9):5099-109. doi: 10.1128/JVI.06991-11. Epub 2012 Feb 22. J Virol. 2012. PMID: 22357277 Free PMC article.
-
The protein kinase double-stranded RNA-dependent (PKR) enhances protection against disease cause by a non-viral pathogen.PLoS Pathog. 2013;9(8):e1003557. doi: 10.1371/journal.ppat.1003557. Epub 2013 Aug 22. PLoS Pathog. 2013. PMID: 23990781 Free PMC article.
-
PKR regulates proliferation, differentiation, and survival of murine hematopoietic stem/progenitor cells.Blood. 2013 Apr 25;121(17):3364-74. doi: 10.1182/blood-2012-09-456400. Epub 2013 Feb 12. Blood. 2013. PMID: 23403623 Free PMC article.
-
Regulatory effects of programmed cell death 4 (PDCD4) protein in interferon (IFN)-stimulated gene expression and generation of type I IFN responses.Mol Cell Biol. 2012 Jul;32(14):2809-22. doi: 10.1128/MCB.00310-12. Epub 2012 May 14. Mol Cell Biol. 2012. PMID: 22586265 Free PMC article.
-
Double-stranded RNA-dependent protein kinase deficiency protects the heart from systolic overload-induced congestive heart failure.Circulation. 2014 Apr 1;129(13):1397-406. doi: 10.1161/CIRCULATIONAHA.113.002209. Epub 2014 Jan 24. Circulation. 2014. PMID: 24463368 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

