Tsetse immune system maturation requires the presence of obligate symbionts in larvae
- PMID: 21655301
- PMCID: PMC3104962
- DOI: 10.1371/journal.pbio.1000619
Tsetse immune system maturation requires the presence of obligate symbionts in larvae
Abstract
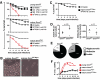
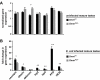
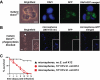
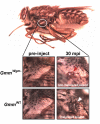
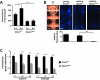
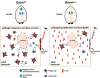
Beneficial microbial symbionts serve important functions within their hosts, including dietary supplementation and maintenance of immune system homeostasis. Little is known about the mechanisms that enable these bacteria to induce specific host phenotypes during development and into adulthood. Here we used the tsetse fly, Glossina morsitans, and its obligate mutualist, Wigglesworthia glossinidia, to investigate the co-evolutionary adaptations that influence the development of host physiological processes. Wigglesworthia is maternally transmitted to tsetse's intrauterine larvae through milk gland secretions. We can produce flies that lack Wigglesworthia (Gmm(Wgm-) yet retain their other symbiotic microbes. Such offspring give rise to adults that exhibit a largely normal phenotype, with the exception being that they are reproductively sterile. Our results indicate that when reared under normal environmental conditions Gmm(Wgm-) adults are also immuno-compromised and highly susceptible to hemocoelic E. coli infections while age-matched wild-type individuals are refractory. Adults that lack Wigglesworthia during larval development exhibit exceptionally compromised cellular and humoral immune responses following microbial challenge, including reduced expression of genes that encode antimicrobial peptides (cecropin and attacin), hemocyte-mediated processes (thioester-containing proteins 2 and 4 and prophenoloxidase), and signal-mediating molecules (inducible nitric oxide synthase). Furthermore, Gmm(Wgm-) adults harbor a reduced population of sessile and circulating hemocytes, a phenomenon that likely results from a significant decrease in larval expression of serpent and lozenge, both of which are associated with the process of early hemocyte differentiation. Our results demonstrate that Wigglesworthia must be present during the development of immature progeny in order for the immune system to function properly in adult tsetse. This phenomenon provides evidence of yet another important physiological adaptation that further anchors the obligate symbiosis between tsetse and Wigglesworthia.
Conflict of interest statement
Serap Aksoy, coauthor on this manuscript, is also Editor-in-Chief of PLoS Neglected Tropical Diseases.
Figures
Comment in
-
Tsetse flies rely on symbiotic Wigglesworthia for immune system development.PLoS Biol. 2011 May;9(5):e1001070. doi: 10.1371/journal.pbio.1001070. Epub 2011 May 31. PLoS Biol. 2011. PMID: 21655305 Free PMC article. No abstract available.
Similar articles
-
Obligate symbionts activate immune system development in the tsetse fly.J Immunol. 2012 Apr 1;188(7):3395-403. doi: 10.4049/jimmunol.1103691. Epub 2012 Feb 24. J Immunol. 2012. PMID: 22368278 Free PMC article.
-
The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly.Appl Environ Microbiol. 2008 Oct;74(19):5965-74. doi: 10.1128/AEM.00741-08. Epub 2008 Aug 8. Appl Environ Microbiol. 2008. PMID: 18689507 Free PMC article.
-
Insight into the transmission biology and species-specific functional capabilities of tsetse (Diptera: glossinidae) obligate symbiont Wigglesworthia.mBio. 2012 Feb 14;3(1):e00240-11. doi: 10.1128/mBio.00240-11. Print 2012. mBio. 2012. PMID: 22334516 Free PMC article.
-
What can a weevil teach a fly, and reciprocally? Interaction of host immune systems with endosymbionts in Glossina and Sitophilus.BMC Microbiol. 2018 Nov 23;18(Suppl 1):150. doi: 10.1186/s12866-018-1278-5. BMC Microbiol. 2018. PMID: 30470176 Free PMC article. Review.
-
Tsetse fly microbiota: form and function.Front Cell Infect Microbiol. 2013 Oct 29;3:69. doi: 10.3389/fcimb.2013.00069. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 24195062 Free PMC article. Review.
Cited by
-
Obligate symbionts activate immune system development in the tsetse fly.J Immunol. 2012 Apr 1;188(7):3395-403. doi: 10.4049/jimmunol.1103691. Epub 2012 Feb 24. J Immunol. 2012. PMID: 22368278 Free PMC article.
-
The holobiont transcriptome of teneral tsetse fly species of varying vector competence.BMC Genomics. 2021 May 31;22(1):400. doi: 10.1186/s12864-021-07729-5. BMC Genomics. 2021. PMID: 34058984 Free PMC article.
-
Antimicrobial peptides and cell processes tracking endosymbiont dynamics.Philos Trans R Soc Lond B Biol Sci. 2016 May 26;371(1695):20150298. doi: 10.1098/rstb.2015.0298. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27160600 Free PMC article. Review.
-
PGRP-LB is a maternally transmitted immune milk protein that influences symbiosis and parasitism in tsetse's offspring.Proc Natl Acad Sci U S A. 2012 Jun 26;109(26):10552-7. doi: 10.1073/pnas.1116431109. Epub 2012 Jun 11. Proc Natl Acad Sci U S A. 2012. PMID: 22689989 Free PMC article.
-
The effect of Wolbachia on gene expression in Drosophila paulistorum and its implications for symbiont-induced host speciation.BMC Genomics. 2019 Jun 7;20(1):465. doi: 10.1186/s12864-019-5816-9. BMC Genomics. 2019. PMID: 31174466 Free PMC article.
References
-
- Moran N. A. Symbiosis. Curr Biol. 2006;16:R866–R871. - PubMed
-
- Wernegreen J. J. Genome evolution in bacterial endosymbionts of insects. Nat Rev Genet. 2002;3:850–861. - PubMed
-
- Aksoy S. Tsetse – a haven for microorganisms. Parasitol Today. 2000;16:114–118. - PubMed
-
- Chen X, Li S, Aksoy S. Concordant evolution of a symbiont with its host insect species: molecular phylogeny of genus Glossina and its bacteriome-associated endosymbiont, Wigglesworthia glossinidia. J Mol Evol. 1999;48:49–58. - PubMed
-
- Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, et al. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglseworthia glossinidia. . Nat Genet. 2002;32:402–407. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

