Protein kinase Cδ is a critical component of Dectin-1 signaling in primary human monocytes
- PMID: 21653233
- PMCID: PMC3157896
- DOI: 10.1189/jlb.0610376
Protein kinase Cδ is a critical component of Dectin-1 signaling in primary human monocytes
Abstract
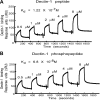
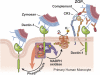
Zymosan, a mimic of fungal pathogens, and its opsonized form (ZOP) are potent stimulators of monocyte NADPH oxidase, resulting in the production of O(2)(.-), which is critical for host defense against fungal and bacterial pathogens and efficient immune responses; however, uncontrolled O(2)(.-) production may contribute to chronic inflammation and tissue injury. Our laboratory has focused on characterizing the signal transduction pathways that regulate NADPH oxidase activity in primary human monocytes. In this study, we examined the involvement of various pattern recognition receptors and found that Dectin-1 is the primary receptor for zymosan stimulation of O(2)(.-) via NADPH oxidase in human monocytes, whereas Dectin-1 and CR3 mediate the activation by ZOP. Further studies identified Syk and Src as important signaling components downstream of Dectin-1 and additionally identified PKCδ as a novel downstream signaling component for zymosan-induced O(2)(.-) as well as phagocytosis. Our results show that Syk and Src association with Dectin-1 is dependent on PKCδ activity and expression and demonstrate direct binding between Dectin-1 and PKCδ. Finally, our data show that PKCδ and Syk but not Src are required for Dectin-1-mediated phagocytosis. Taken together, our data identify Dectin-1 as the major PRR for zymosan in primary human monocytes and identify PKCδ as a novel downstream signaling kinase for Dectin-1-mediated regulation of monocyte NADPH oxidase and zymosan phagocytosis.
Figures
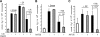
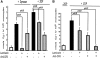

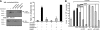




Similar articles
-
Regulation of cytosolic phospholipase A2 activation and cyclooxygenase 2 expression in macrophages by the beta-glucan receptor.J Biol Chem. 2006 Mar 3;281(9):5506-14. doi: 10.1074/jbc.M509824200. Epub 2006 Jan 3. J Biol Chem. 2006. PMID: 16407295
-
Costimulation of dectin-1 and DC-SIGN triggers the arachidonic acid cascade in human monocyte-derived dendritic cells.J Immunol. 2008 Apr 15;180(8):5727-36. doi: 10.4049/jimmunol.180.8.5727. J Immunol. 2008. PMID: 18390758
-
Dissimilar and similar functional properties of complement receptor-3 in microglia and macrophages in combating yeast pathogens by phagocytosis.Glia. 2010 May;58(7):823-30. doi: 10.1002/glia.20966. Glia. 2010. PMID: 20091776
-
The role of Dectin-1 in the host defence against fungal infections.Curr Opin Microbiol. 2011 Aug;14(4):392-9. doi: 10.1016/j.mib.2011.07.001. Epub 2011 Jul 29. Curr Opin Microbiol. 2011. PMID: 21803640 Review.
-
CARD9 Syk-dependent and Raf-1 Syk-independent signaling pathways in target recognition of Candida albicans by Dectin-1.Eur J Clin Microbiol Infect Dis. 2011 Mar;30(3):303-5. doi: 10.1007/s10096-010-1103-z. Epub 2010 Nov 25. Eur J Clin Microbiol Infect Dis. 2011. PMID: 21108038 Review.
Cited by
-
Phosphorylation of PFKL regulates metabolic reprogramming in macrophages following pattern recognition receptor activation.Nat Commun. 2024 Jul 31;15(1):6438. doi: 10.1038/s41467-024-50104-7. Nat Commun. 2024. PMID: 39085210 Free PMC article.
-
PCSK9 stimulates Syk, PKCδ, and NF-κB, leading to atherosclerosis progression independently of LDL receptor.Nat Commun. 2024 Mar 30;15(1):2789. doi: 10.1038/s41467-024-46336-2. Nat Commun. 2024. PMID: 38555386 Free PMC article.
-
New Insights into the Pro-Inflammatory and Osteoclastogenic Profile of Circulating Monocytes in Osteoarthritis Patients.Int J Mol Sci. 2024 Jan 30;25(3):1710. doi: 10.3390/ijms25031710. Int J Mol Sci. 2024. PMID: 38338988 Free PMC article.
-
Candida Administration Worsens Neutrophil Extracellular Traps in Renal Ischemia Reperfusion Injury Mice: An Impact of Gut Fungi on Acute Kidney Injury.J Innate Immun. 2022;14(5):502-517. doi: 10.1159/000521633. Epub 2022 Jan 28. J Innate Immun. 2022. PMID: 35093955 Free PMC article.
-
Mitofusin 2 Deficiency Causes Pro-Inflammatory Effects in Human Primary Macrophages.Front Immunol. 2021 Aug 12;12:723683. doi: 10.3389/fimmu.2021.723683. eCollection 2021. Front Immunol. 2021. PMID: 34456930 Free PMC article.
References
-
- Cathcart M. K., McNally A. K., Morel D. W., Chisolm G. M., III (1989) Superoxide anion participation in human monocyte-mediated oxidation of low-density lipoprotein and conversion of low-density lipoprotein to a cytotoxin. J. Immunol. 142, 1963–1969 - PubMed
-
- Bey E. A., Cathcart M. K. (2000) In vitro knockout of human p47phox blocks superoxide anion production and LDL oxidation by activated human monocytes. J. Lipid Res. 41, 489–495 - PubMed
-
- Li Q., Subbulakshmi V., Fields A. P., Murray N. R., Cathcart M. K. (1999) Protein kinase Cα regulates human monocyte O-2 production and low density lipoprotein lipid oxidation. J. Biol. Chem. 274, 3764–3771 - PubMed
-
- Li Q., Cathcart M. K. (1997) Selective inhibition of cytosolic phospholipase A2 in activated human monocytes. Regulation of superoxide anion production and low density lipoprotein oxidation. J. Biol. Chem. 272, 2404–2411 - PubMed
-
- Cathcart M. K. (2004) Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 24, 23–28 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

