Heterotrimeric G proteins, focal adhesion kinase, and endothelial barrier function
- PMID: 21640127
- PMCID: PMC3214251
- DOI: 10.1016/j.mvr.2011.05.004
Heterotrimeric G proteins, focal adhesion kinase, and endothelial barrier function
Abstract
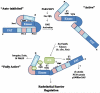
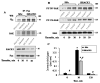
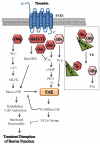
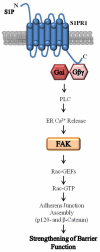
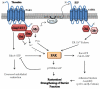
Ligands by binding to G protein coupled receptors (GPCRs) stimulate dissociation of heterotrimeric G proteins into Gα and Gβγ subunits. Released Gα and Gβγ subunits induce discrete signaling cues that differentially regulate focal adhesion kinase (FAK) activity and endothelial barrier function. Activation of G proteins downstream of receptors such as protease activated receptor 1 (PAR1) and histamine receptors rapidly increases endothelial permeability which reverses naturally within the following 1-2 h. However, activation of G proteins coupled to the sphingosine-1-phosphate receptor 1 (S1P1) signal cues that enhance basal barrier endothelial function and restore endothelial barrier function following the increase in endothelial permeability by edemagenic agents. Intriguingly, both PAR1 and S1P1 activation stimulates FAK activity, which associates with alteration in endothelial barrier function by these agonists. In this review, we focus on the role of the G protein subunits downstream of PAR1 and S1P1 in regulating FAK activity and endothelial barrier function.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
The G protein betagamma subunit mediates reannealing of adherens junctions to reverse endothelial permeability increase by thrombin.J Exp Med. 2009 Nov 23;206(12):2761-77. doi: 10.1084/jem.20090652. Epub 2009 Nov 16. J Exp Med. 2009. PMID: 19917775 Free PMC article.
-
Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells.Circ Res. 2008 Nov 7;103(10):1164-72. doi: 10.1161/01.RES.0000338501.84810.51. Epub 2008 Oct 10. Circ Res. 2008. PMID: 18849324 Free PMC article.
-
Role of FAK in S1P-regulated endothelial permeability.Microvasc Res. 2012 Jan;83(1):22-30. doi: 10.1016/j.mvr.2011.08.012. Epub 2011 Sep 5. Microvasc Res. 2012. PMID: 21925517 Free PMC article. Review.
-
Transforming growth factor-beta1 effects on endothelial monolayer permeability involve focal adhesion kinase/Src.Am J Respir Cell Mol Biol. 2007 Oct;37(4):485-93. doi: 10.1165/rcmb.2006-0439OC. Epub 2007 Jun 21. Am J Respir Cell Mol Biol. 2007. PMID: 17585111 Free PMC article.
-
Neutrophil transmigration, focal adhesion kinase and endothelial barrier function.Microvasc Res. 2012 Jan;83(1):82-8. doi: 10.1016/j.mvr.2011.06.015. Epub 2011 Aug 16. Microvasc Res. 2012. PMID: 21864543 Free PMC article. Review.
Cited by
-
Mechanisms regulating endothelial permeability.Pulm Circ. 2014 Dec;4(4):535-51. doi: 10.1086/677356. Pulm Circ. 2014. PMID: 25610592 Free PMC article. Review.
-
Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions.Int J Biol Sci. 2013 Sep 23;9(9):966-79. doi: 10.7150/ijbs.6996. eCollection 2013. Int J Biol Sci. 2013. PMID: 24155670 Free PMC article.
-
Post-translational modifications of S1PR1 and endothelial barrier regulation.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Sep;1865(9):158760. doi: 10.1016/j.bbalip.2020.158760. Epub 2020 Jun 22. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 32585303 Free PMC article. Review.
-
Extracellular matrix stiffness-The central cue for skin fibrosis.Front Mol Biosci. 2023 Mar 8;10:1132353. doi: 10.3389/fmolb.2023.1132353. eCollection 2023. Front Mol Biosci. 2023. PMID: 36968277 Free PMC article. Review.
-
The transient receptor potential vanilloid 4 (TRPV4) ion channel mediates protease activated receptor 1 (PAR1)-induced vascular hyperpermeability.Lab Invest. 2020 Aug;100(8):1057-1067. doi: 10.1038/s41374-020-0430-7. Epub 2020 Apr 27. Lab Invest. 2020. PMID: 32341518 Free PMC article.
References
-
- Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem. 1997;272:15442–51. - PubMed
-
- Ahmmed GU, Malik AB. Functional role of TRPC channels in the regulation of endothelial permeability. Pflugers Arch. 2005;451:131–42. - PubMed
-
- Alessandro R, et al. Endothelial cell spreading on type IV collagen and spreading-induced FAK phosphorylation is regulated by Ca2+ influx. Biochem Biophys Res Commun. 1998;248:635–40. - PubMed
-
- Allende ML, et al. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

