Membrane organization and lipid rafts
- PMID: 21628426
- PMCID: PMC3179338
- DOI: 10.1101/cshperspect.a004697
Membrane organization and lipid rafts
Abstract
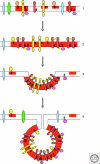
Cell membranes are composed of a lipid bilayer, containing proteins that span the bilayer and/or interact with the lipids on either side of the two leaflets. Although recent advances in lipid analytics show that membranes in eukaryotic cells contain hundreds of different lipid species, the function of this lipid diversity remains enigmatic. The basic structure of cell membranes is the lipid bilayer, composed of two apposing leaflets, forming a two-dimensional liquid with fascinating properties designed to perform the functions cells require. To coordinate these functions, the bilayer has evolved the propensity to segregate its constituents laterally. This capability is based on dynamic liquid-liquid immiscibility and underlies the raft concept of membrane subcompartmentalization. This principle combines the potential for sphingolipid-cholesterol self-assembly with protein specificity to focus and regulate membrane bioactivity. Here we will review the emerging principles of membrane architecture with special emphasis on lipid organization and domain formation.
Figures

Similar articles
-
Lipid rafts as a membrane-organizing principle.Science. 2010 Jan 1;327(5961):46-50. doi: 10.1126/science.1174621. Science. 2010. PMID: 20044567 Review.
-
Lipid rafts as functional heterogeneity in cell membranes.Biochem Soc Trans. 2009 Oct;37(Pt 5):955-60. doi: 10.1042/BST0370955. Biochem Soc Trans. 2009. PMID: 19754431 Review.
-
Is a fluid-mosaic model of biological membranes fully relevant? Studies on lipid organization in model and biological membranes.Cell Mol Biol Lett. 2003;8(1):147-59. Cell Mol Biol Lett. 2003. PMID: 12655369
-
Partitioning of membrane molecules between raft and non-raft domains: insights from model-membrane studies.Biochim Biophys Acta. 2005 Dec 30;1746(3):193-202. doi: 10.1016/j.bbamcr.2005.09.003. Epub 2005 Sep 23. Biochim Biophys Acta. 2005. PMID: 16271405 Review.
-
Thermosensing via transmembrane protein-lipid interactions.Biochim Biophys Acta. 2015 Sep;1848(9):1757-64. doi: 10.1016/j.bbamem.2015.04.005. Epub 2015 Apr 20. Biochim Biophys Acta. 2015. PMID: 25906947 Review.
Cited by
-
Differential recognition of lipid domains by two Gb3-binding lectins.Sci Rep. 2020 Jun 16;10(1):9752. doi: 10.1038/s41598-020-66522-8. Sci Rep. 2020. PMID: 32546842 Free PMC article.
-
A single native ganglioside GM1-binding site is sufficient for cholera toxin to bind to cells and complete the intoxication pathway.mBio. 2012 Oct 30;3(6):e00401-12. doi: 10.1128/mBio.00401-12. mBio. 2012. PMID: 23111873 Free PMC article.
-
Histoplasma capsulatum-Induced Cytokine Secretion in Lung Epithelial Cells Is Dependent on Host Integrins, Src-Family Kinase Activation, and Membrane Raft Recruitment.Front Microbiol. 2016 Apr 22;7:580. doi: 10.3389/fmicb.2016.00580. eCollection 2016. Front Microbiol. 2016. PMID: 27148251 Free PMC article.
-
Eisosomes are dynamic plasma membrane domains showing pil1-lsp1 heteroligomer binding equilibrium.Biophys J. 2015 Apr 7;108(7):1633-1644. doi: 10.1016/j.bpj.2015.02.011. Biophys J. 2015. PMID: 25863055 Free PMC article.
-
Overexpressing lipid raft protein STOML2 modulates the tumor microenvironment via NF-κB signaling in colorectal cancer.Cell Mol Life Sci. 2024 Jan 12;81(1):39. doi: 10.1007/s00018-023-05105-y. Cell Mol Life Sci. 2024. PMID: 38214751 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
