Nucleosomes protect DNA from DNA methylation in vivo and in vitro
- PMID: 21622955
- PMCID: PMC3167622
- DOI: 10.1093/nar/gkr263
Nucleosomes protect DNA from DNA methylation in vivo and in vitro
Abstract
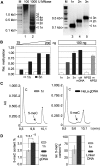
Positioned nucleosomes limit the access of proteins to DNA. However, the impact of nucleosomes on DNA methylation in vitro and in vivo is poorly understood. Here, we performed a detailed analysis of nucleosome binding and nucleosomal DNA methylation by the de novo methyltransferases. We show that compared to linker DNA, nucleosomal DNA is largely devoid of CpG methylation. ATP-dependent chromatin remodelling frees nucleosomal CpG dinucleotides and renders the remodelled nucleosome a 2-fold better substrate for Dnmt3a methyltransferase compared to free DNA. These results reflect the situation in vivo, as quantification of nucleosomal DNA methylation levels in HeLa cells shows a 2-fold decrease of nucleosomal DNA methylation levels compared to linker DNA. Our findings suggest that nucleosomal positions are stably maintained in vivo and nucleosomal occupancy is a major determinant of global DNA methylation patterns in vivo.
Figures




Similar articles
-
Structure of nucleosome-bound DNA methyltransferases DNMT3A and DNMT3B.Nature. 2020 Oct;586(7827):151-155. doi: 10.1038/s41586-020-2747-1. Epub 2020 Sep 23. Nature. 2020. PMID: 32968275 Free PMC article.
-
Characterization of Dnmt1 Binding and DNA Methylation on Nucleosomes and Nucleosomal Arrays.PLoS One. 2015 Oct 23;10(10):e0140076. doi: 10.1371/journal.pone.0140076. eCollection 2015. PLoS One. 2015. PMID: 26496704 Free PMC article.
-
Distinct DNA methylation activity of Dnmt3a and Dnmt3b towards naked and nucleosomal DNA.J Biochem. 2006 Mar;139(3):503-15. doi: 10.1093/jb/mvj044. J Biochem. 2006. PMID: 16567415
-
Chromatin remodelling: the industrial revolution of DNA around histones.Nat Rev Mol Cell Biol. 2006 Jun;7(6):437-47. doi: 10.1038/nrm1945. Nat Rev Mol Cell Biol. 2006. PMID: 16723979 Review.
-
Targeting chromatin remodelers: signals and search mechanisms.Biochim Biophys Acta. 2011 Sep;1809(9):497-508. doi: 10.1016/j.bbagrm.2011.06.005. Epub 2011 Jun 16. Biochim Biophys Acta. 2011. PMID: 21704204 Review.
Cited by
-
Coevolution of the CDCA7-HELLS ICF-related nucleosome remodeling complex and DNA methyltransferases.Elife. 2023 Sep 28;12:RP86721. doi: 10.7554/eLife.86721. Elife. 2023. PMID: 37769127 Free PMC article.
-
Understanding the connection between epigenetic DNA methylation and nucleosome positioning from computer simulations.PLoS Comput Biol. 2013;9(11):e1003354. doi: 10.1371/journal.pcbi.1003354. Epub 2013 Nov 21. PLoS Comput Biol. 2013. PMID: 24278005 Free PMC article.
-
Nucleosomes are enriched at the boundaries of hypomethylated regions (HMRs) in mouse dermal fibroblasts and keratinocytes.Epigenetics Chromatin. 2014 Dec 2;7(1):34. doi: 10.1186/1756-8935-7-34. eCollection 2014. Epigenetics Chromatin. 2014. PMID: 25506399 Free PMC article.
-
MeCP2 binds to nucleosome free (linker DNA) regions and to H3K9/H3K27 methylated nucleosomes in the brain.Nucleic Acids Res. 2012 Apr;40(7):2884-97. doi: 10.1093/nar/gkr1066. Epub 2011 Dec 5. Nucleic Acids Res. 2012. PMID: 22144686 Free PMC article.
-
A Nucleosome Bridging Mechanism for Activation of a Maintenance DNA Methyltransferase.Mol Cell. 2019 Jan 3;73(1):73-83.e6. doi: 10.1016/j.molcel.2018.10.006. Epub 2018 Nov 8. Mol Cell. 2019. PMID: 30415948 Free PMC article.
References
-
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002;3:415–428. - PubMed
-
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. - PubMed
-
- Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat. Rev. Genet. 2002;3:662–673. - PubMed
-
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem. Sci. 2006;31:89–97. - PubMed
-
- Freitag M, Selker EU. Controlling DNA methylation: many roads to one modification. Curr. Opin. Genet. Dev. 2005;15:191–199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

