A two-site mechanism for the inhibition of IAPP amyloidogenesis by zinc
- PMID: 21616080
- PMCID: PMC3115507
- DOI: 10.1016/j.jmb.2011.05.015
A two-site mechanism for the inhibition of IAPP amyloidogenesis by zinc
Abstract
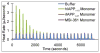
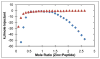
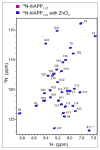
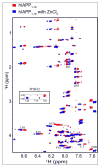
Human islet amyloid polypeptide (hIAPP) is a highly amyloidogenic protein co-secreted with insulin in response to glucose levels. The formation of hIAPP amyloid plaques near islet cells has been linked to the death of insulin-secreting β-cells in humans and the progression of type II diabetes. Since both healthy individuals and those with type II diabetes produce and secrete hIAPP, it is reasonable to look for factors involved in storing hIAPP and preventing amyloidosis. We have previously shown that zinc inhibits the formation of insoluble amyloid plaques of hIAPP; however, there remains significant ambiguity in the underlying mechanisms. In this study, we show that zinc binds unaggregated hIAPP at micromolar concentrations similar to those found in the extracellular environment. By contrast, the fibrillar amyloid form of hIAPP has low affinity for zinc. The binding stoichiometry obtained from isothermal titration calorimetry experiments indicates that zinc favors the formation of hIAPP hexamers. High-resolution NMR structures of hIAPP bound to zinc reveal changes in the electron environment along residues that would be located along one face of the amphipathic hIAPP α-helix proposed as an intermediate for amyloid formation. Results from electrospray ionization mass spectroscopy investigations showed that a single zinc atom is predominantly bound to hIAPP and revealed that zinc inhibits the formation of the dimer. At higher concentrations of zinc, a second zinc atom binds to hIAPP, suggesting the presence of a low-affinity secondary binding site. Combined, these results suggest that zinc promotes the formation of oligomers while creating an energetic barrier for the formation of amyloid fibers.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

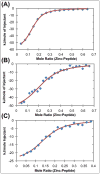
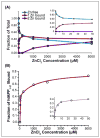
 ), zinc has one apparent binding site at sub- stoichiometric ratios of 0.136 zinc to 1 hIAPP1–19. In the presence of 30% TFE (
), zinc has one apparent binding site at sub- stoichiometric ratios of 0.136 zinc to 1 hIAPP1–19. In the presence of 30% TFE (
 ) the peptide adopts an α-helical conformation, causing a shift in the apparent binding stoichiometry of the primary binding site to 0.24 zinc to 1 hIAPP1–19 and the addition of an endothermic process at higher concentrations of zinc.
) the peptide adopts an α-helical conformation, causing a shift in the apparent binding stoichiometry of the primary binding site to 0.24 zinc to 1 hIAPP1–19 and the addition of an endothermic process at higher concentrations of zinc.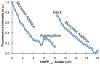


Similar articles
-
Investigation of the effects of two major secretory granules components, insulin and zinc, on human-IAPP amyloid aggregation and membrane damage.Chem Phys Lipids. 2021 Jul;237:105083. doi: 10.1016/j.chemphyslip.2021.105083. Epub 2021 Apr 19. Chem Phys Lipids. 2021. PMID: 33887213
-
Role of zinc in human islet amyloid polypeptide aggregation.J Am Chem Soc. 2010 Jul 7;132(26):8973-83. doi: 10.1021/ja1007867. J Am Chem Soc. 2010. PMID: 20536124 Free PMC article.
-
Analysis of the Role of the Conserved Disulfide in Amyloid Formation by Human Islet Amyloid Polypeptide in Homogeneous and Heterogeneous Environments.Biochemistry. 2018 May 29;57(21):3065-3074. doi: 10.1021/acs.biochem.8b00017. Epub 2018 May 14. Biochemistry. 2018. PMID: 29697253 Free PMC article.
-
Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology.Biochim Biophys Acta. 2001 Nov 29;1537(3):179-203. doi: 10.1016/s0925-4439(01)00078-3. Biochim Biophys Acta. 2001. PMID: 11731221 Review.
-
Human islet amyloid polypeptide (hIAPP) - a curse in type II diabetes mellitus: insights from structure and toxicity studies.Biol Chem. 2020 Sep 4;402(2):133-153. doi: 10.1515/hsz-2020-0174. Print 2021 Jan 27. Biol Chem. 2020. PMID: 33544470 Review.
Cited by
-
Human islet amyloid polypeptide: A therapeutic target for the management of type 2 diabetes mellitus.J Pharm Anal. 2022 Aug;12(4):556-569. doi: 10.1016/j.jpha.2022.04.001. Epub 2022 Apr 7. J Pharm Anal. 2022. PMID: 36105173 Free PMC article. Review.
-
Metal-dependent hormone function: the emerging interdisciplinary field of metalloendocrinology.Metallomics. 2019 Jan 23;11(1):85-110. doi: 10.1039/c8mt00221e. Metallomics. 2019. PMID: 30270362 Free PMC article. Review.
-
Zinc and pH modulate the ability of insulin to inhibit aggregation of islet amyloid polypeptide.Commun Biol. 2024 Jun 27;7(1):776. doi: 10.1038/s42003-024-06388-y. Commun Biol. 2024. PMID: 38937578 Free PMC article.
-
The potential role of human islet amyloid polypeptide in type 2 diabetes mellitus and Alzheimer's diseases.Diabetol Metab Syndr. 2023 May 13;15(1):101. doi: 10.1186/s13098-023-01082-1. Diabetol Metab Syndr. 2023. PMID: 37173803 Free PMC article. Review.
-
Human islet amyloid polypeptide at the air-aqueous interface: a Langmuir monolayer approach.J R Soc Interface. 2012 Nov 7;9(76):3118-28. doi: 10.1098/rsif.2012.0368. Epub 2012 Jul 11. J R Soc Interface. 2012. PMID: 22787008 Free PMC article.
References
-
- Leonardi O, Mints G, Hussain MA. Beta-cell apoptosis in the pathogenesis of human type 2 diabetes mellitus. Eur J Endocrinol. 2003;149:99–102. - PubMed
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. - PubMed
-
- Dekoning EJP, Morris ER, Hofhuis FMA, Posthuma G, Hoppener JWM, Morris JF, Capel PJA, Clark A, Verbeek JS. Intracellular and extracellular amyloid fibrils are formed in cultured pancreatic-islets of transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1994;91:8467–8471. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

