Identification of Cd101 as a susceptibility gene for Novosphingobium aromaticivorans-induced liver autoimmunity
- PMID: 21613619
- PMCID: PMC3134939
- DOI: 10.4049/jimmunol.1003525
Identification of Cd101 as a susceptibility gene for Novosphingobium aromaticivorans-induced liver autoimmunity
Abstract
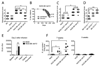
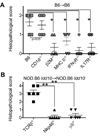
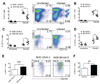
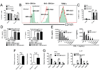
Environmental and genetic factors define the susceptibility of an individual to autoimmune disease. Although common genetic pathways affect general immunological tolerance mechanisms in autoimmunity, the effects of such genes could vary under distinct immune challenges within different tissues. In this study, we demonstrate this by observing that autoimmune type 1 diabetes-protective haplotypes at the insulin-dependent diabetes susceptibility region 10 (Idd10) introgressed from chromosome 3 of C57BL/6 (B6) and A/J mice onto the NOD background increase the severity of autoimmune primary biliary cirrhosis induced by infection with Novosphingobium aromaticivorans, a ubiquitous alphaproteobacterium, when compared with mice having the NOD and NOD.CAST Idd10 type 1 diabetes-susceptible haplotypes. Substantially increased liver pathology in mice having the B6 and A/J Idd10 haplotypes correlates with reduced expression of CD101 on dendritic cells, macrophages, and granulocytes following infection, delayed clearance of N. aromaticivorans, and the promotion of overzealous IFN-γ- and IL-17-dominated T cell responses essential for the adoptive transfer of liver lesions. CD101-knockout mice generated on the B6 background also exhibit substantially more severe N. aromaticivorans-induced liver disease correlating with increased IFN-γ and IL-17 responses compared with wild-type mice. These data strongly support the hypothesis that allelic variation of the Cd101 gene, located in the Idd10 region, alters the severity of liver autoimmunity induced by N. aromaticivorans.
Figures


Similar articles
-
Escherichia coli infection induces autoimmune cholangitis and anti-mitochondrial antibodies in non-obese diabetic (NOD).B6 (Idd10/Idd18) mice.Clin Exp Immunol. 2014 Feb;175(2):192-201. doi: 10.1111/cei.12224. Clin Exp Immunol. 2014. PMID: 24128311 Free PMC article.
-
Genetic and functional data identifying Cd101 as a type 1 diabetes (T1D) susceptibility gene in nonobese diabetic (NOD) mice.PLoS Genet. 2019 Jun 14;15(6):e1008178. doi: 10.1371/journal.pgen.1008178. eCollection 2019 Jun. PLoS Genet. 2019. PMID: 31199784 Free PMC article.
-
Liver autoimmunity triggered by microbial activation of natural killer T cells.Cell Host Microbe. 2008 May 15;3(5):304-15. doi: 10.1016/j.chom.2008.03.009. Cell Host Microbe. 2008. PMID: 18474357 Free PMC article.
-
Environmental basis of primary biliary cholangitis.Exp Biol Med (Maywood). 2018 Jan;243(2):184-189. doi: 10.1177/1535370217748893. Epub 2018 Jan 7. Exp Biol Med (Maywood). 2018. PMID: 29307284 Free PMC article. Review.
-
Etiopathogenesis of autoimmune hepatitis.J Autoimmun. 2018 Dec;95:133-143. doi: 10.1016/j.jaut.2018.10.020. Epub 2018 Oct 29. J Autoimmun. 2018. PMID: 30385083 Review.
Cited by
-
Escherichia coli infection induces autoimmune cholangitis and anti-mitochondrial antibodies in non-obese diabetic (NOD).B6 (Idd10/Idd18) mice.Clin Exp Immunol. 2014 Feb;175(2):192-201. doi: 10.1111/cei.12224. Clin Exp Immunol. 2014. PMID: 24128311 Free PMC article.
-
Impact of Microbes on the Pathogenesis of Primary Biliary Cirrhosis (PBC) and Primary Sclerosing Cholangitis (PSC).Int J Mol Sci. 2016 Nov 9;17(11):1864. doi: 10.3390/ijms17111864. Int J Mol Sci. 2016. PMID: 27834858 Free PMC article. Review.
-
CD101 inhibits the expansion of colitogenic T cells.Mucosal Immunol. 2016 Sep;9(5):1205-17. doi: 10.1038/mi.2015.139. Epub 2016 Jan 27. Mucosal Immunol. 2016. PMID: 26813346 Free PMC article.
-
Bile Acids and Microbiota: Multifaceted and Versatile Regulators of the Liver-Gut Axis.Int J Mol Sci. 2021 Jan 30;22(3):1397. doi: 10.3390/ijms22031397. Int J Mol Sci. 2021. PMID: 33573273 Free PMC article. Review.
-
Potential Roles for Infectious Agents in the Pathophysiology of Primary Biliary Cirrhosis: What's New?Curr Infect Dis Rep. 2013 Feb;15(1):14-24. doi: 10.1007/s11908-012-0304-2. Curr Infect Dis Rep. 2013. PMID: 23188623
References
-
- Rioux JD, Abbas AK. Paths to understanding the genetic basis of autoimmune disease. Nature. 2005;435:584–589. - PubMed
-
- Bjorses P, Aaltonen J, Horelli-Kuitunen N, Yaspo ML, Peltonen L. Gene defect behind APECED: a new clue to autoimmunity. Hum. Mol. Genet. 1998;7:1547–1553. - PubMed
-
- Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat. Rev Immunol. 2002;2:11–19. - PubMed
-
- He XS, Ansari AA, Ridgway WM, Coppel RL, Gershwin ME. New insights to the immunopathology and autoimmune responses in primary biliary cirrhosis. Cell Immunol. 2006;239:1–13. - PubMed
-
- Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

