Long-term outcome of individuals treated with oral insulin: diabetes prevention trial-type 1 (DPT-1) oral insulin trial
- PMID: 21610124
- PMCID: PMC3120180
- DOI: 10.2337/dc11-0523
Long-term outcome of individuals treated with oral insulin: diabetes prevention trial-type 1 (DPT-1) oral insulin trial
Abstract
Objective: To evaluate the long-term intervention effects of oral insulin on the development of type 1 diabetes and to assess the rate of progression to type 1 diabetes before and after oral insulin treatment was stopped in the Diabetes Prevention Trial-Type 1 (DPT-1).
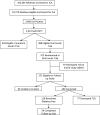
Research design and methods: The follow-up included subjects who participated in the early intervention of oral insulin (1994-2003) to prevent or delay type 1 diabetes. A telephone survey was conducted in 2009 to determine whether diabetes had been diagnosed and, if not, an oral glucose tolerance test (OGTT), hemoglobin A1c (HbA1c), and autoantibody levels were obtained on all subjects who agreed to participate.
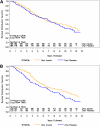
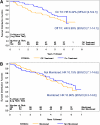
Results: Of 372 subjects randomized, 97 developed type 1 diabetes before follow-up; 75% of the remaining 275 subjects were contacted. In the interim, 77 subjects had been diagnosed with type 1 diabetes and 54 of the remainder have had an OGTT; 10 of these were diagnosed with type 1 diabetes, subsequently. Among individuals meeting the original criteria for insulin autoantibodies (IAAs) (≥80 nU/mL), the overall benefit of oral insulin remained significant (P=0.05). However, the hazard rate in this group increased (from 6.4% [95% CI 4.5-9.1] to 10.0% [7.1-14.1]) after cessation of therapy, which approximated the rate of individuals treated with placebo (10.2% [7.1-14.6]).
Conclusions: Overall, the oral insulin treatment effect in individuals with confirmed IAA≥80 nU/mL appeared to be maintained with additional follow-up; however, once therapy stopped, the rate of developing diabetes in the oral insulin group increased to a rate similar to that in the placebo group.
Figures

Similar articles
-
Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial--Type 1.Diabetes Care. 2005 May;28(5):1068-76. doi: 10.2337/diacare.28.5.1068. Diabetes Care. 2005. PMID: 15855569 Clinical Trial.
-
Effect of Oral Insulin on Prevention of Diabetes in Relatives of Patients With Type 1 Diabetes: A Randomized Clinical Trial.JAMA. 2017 Nov 21;318(19):1891-1902. doi: 10.1001/jama.2017.17070. JAMA. 2017. PMID: 29164254 Free PMC article. Clinical Trial.
-
Effect of oral insulin on insulin autoantibody levels in the Diabetes Prevention Trial Type 1 oral insulin study.Diabetologia. 2007 Aug;50(8):1603-6. doi: 10.1007/s00125-007-0694-0. Epub 2007 Jun 22. Diabetologia. 2007. PMID: 17583798 Clinical Trial.
-
Insulin and oral agents for managing cystic fibrosis-related diabetes.Cochrane Database Syst Rev. 2016 Apr 18;4:CD004730. doi: 10.1002/14651858.CD004730.pub4. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2020 Oct 19;10:CD004730. doi: 10.1002/14651858.CD004730.pub5 PMID: 27087121 Updated. Review.
-
The metabolic progression to type 1 diabetes as indicated by serial oral glucose tolerance testing in the Diabetes Prevention Trial-type 1.Diabetes. 2012 Jun;61(6):1331-7. doi: 10.2337/db11-1660. Diabetes. 2012. PMID: 22618768 Free PMC article. Review. No abstract available.
Cited by
-
Preexisting insulin autoantibodies predict efficacy of otelixizumab in preserving residual β-cell function in recent-onset type 1 diabetes.Diabetes Care. 2015 Apr;38(4):644-51. doi: 10.2337/dc14-1575. Epub 2015 Jan 12. Diabetes Care. 2015. PMID: 25583753 Free PMC article. Clinical Trial.
-
Efficacy and safety of oral insulin versus placebo for patients with diabetes mellitus: A systematic review and meta-analysis.Indian J Pharmacol. 2022 Jul-Aug;54(4):244-252. doi: 10.4103/ijp.ijp_1135_20. Indian J Pharmacol. 2022. PMID: 36204807 Free PMC article.
-
Primary and secondary prevention of Type 1 diabetes.Diabet Med. 2013 Feb;30(2):161-9. doi: 10.1111/dme.12100. Diabet Med. 2013. PMID: 23231526 Free PMC article. Review.
-
Immune therapy in type 1 diabetes mellitus.Nat Rev Endocrinol. 2013 Feb;9(2):92-103. doi: 10.1038/nrendo.2012.237. Epub 2013 Jan 8. Nat Rev Endocrinol. 2013. PMID: 23296174 Review.
-
Where, How, and When: Positioning Posttranslational Modification Within Type 1 Diabetes Pathogenesis.Curr Diab Rep. 2016 Jul;16(7):63. doi: 10.1007/s11892-016-0752-4. Curr Diab Rep. 2016. PMID: 27168063 Free PMC article. Review.
References
-
- Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial–Type 1. Diabetes Care 2005;28:1068–1076 - PubMed
-
- Diabetes Prevention Trial–Type 1 Diabetes Study Group Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002;346:1685–1691 - PubMed
-
- Wang J, Miao D, Babu S, et al. Prevalence of autoantibody-negative diabetes is not rare at all ages and increases with older age and obesity. J Clin Endocrinol Metab 2007;92:88–92 - PubMed
-
- Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

