Defining the RNA internal loops preferred by benzimidazole derivatives via 2D combinatorial screening and computational analysis
- PMID: 21604752
- PMCID: PMC3126894
- DOI: 10.1021/ja200212b
Defining the RNA internal loops preferred by benzimidazole derivatives via 2D combinatorial screening and computational analysis
Abstract
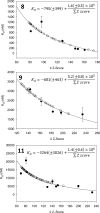
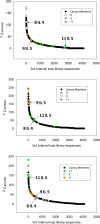
RNA is an important therapeutic target; however, RNA targets are generally underexploited due to a lack of understanding of the small molecules that bind RNA and the RNA motifs that bind small molecules. Herein, we describe the identification of the RNA internal loops derived from a 4096 member 3 × 3 nucleotide loop library that are the most specific and highest affinity binders to a series of four designer, druglike benzimidazoles. These studies establish a potentially general protocol to define the highest affinity and most specific RNA motif targets for heterocyclic small molecules. Such information could be used to target functionally important RNAs in genomic sequence.
Figures


Similar articles
-
Two-dimensional combinatorial screening of a bacterial rRNA A-site-like motif library: defining privileged asymmetric internal loops that bind aminoglycosides.Biochemistry. 2010 Mar 9;49(9):1833-42. doi: 10.1021/bi901998m. Biochemistry. 2010. PMID: 20108982 Free PMC article.
-
Probing a 2-aminobenzimidazole library for binding to RNA internal loops via two-dimensional combinatorial screening.ACS Chem Biol. 2012 Nov 16;7(11):1902-9. doi: 10.1021/cb300213g. Epub 2012 Sep 14. ACS Chem Biol. 2012. PMID: 22958065 Free PMC article.
-
Two-dimensional combinatorial screening identifies specific 6'-acylated kanamycin A- and 6'-acylated neamine-RNA hairpin interactions.Biochemistry. 2008 Dec 2;47(48):12670-9. doi: 10.1021/bi8012615. Biochemistry. 2008. PMID: 18991404 Free PMC article.
-
Nucleic acid encoding to program self-assembly in chemical biology.Chem Soc Rev. 2008 Jul;37(7):1330-6. doi: 10.1039/b706610b. Epub 2008 Apr 17. Chem Soc Rev. 2008. PMID: 18568159 Review.
-
REPSA: general combinatorial approach for identifying preferred ligand-DNA binding sequences.Methods. 2007 Jun;42(2):118-27. doi: 10.1016/j.ymeth.2006.09.008. Methods. 2007. PMID: 17472894 Review.
Cited by
-
How We Think about Targeting RNA with Small Molecules.J Med Chem. 2020 Sep 10;63(17):8880-8900. doi: 10.1021/acs.jmedchem.9b01927. Epub 2020 Mar 26. J Med Chem. 2020. PMID: 32212706 Free PMC article.
-
Studying a Drug-like, RNA-Focused Small Molecule Library Identifies Compounds That Inhibit RNA Toxicity in Myotonic Dystrophy.ACS Chem Biol. 2015 Dec 18;10(12):2706-15. doi: 10.1021/acschembio.5b00430. Epub 2015 Sep 28. ACS Chem Biol. 2015. PMID: 26414664 Free PMC article.
-
A pH Sensitive High-Throughput Assay for miRNA Binding of a Peptide-Aminoglycoside (PA) Library.PLoS One. 2015 Dec 11;10(12):e0144251. doi: 10.1371/journal.pone.0144251. eCollection 2015. PLoS One. 2015. PMID: 26656788 Free PMC article.
-
Method for Identifying Sequence Motifs in Pre-miRNAs for Small-Molecule Binding.ACS Chem Biol. 2022 Oct 21;17(10):2817-2827. doi: 10.1021/acschembio.2c00452. Epub 2022 Sep 23. ACS Chem Biol. 2022. PMID: 36150699 Free PMC article.
-
Identifying the preferred RNA motifs and chemotypes that interact by probing millions of combinations.Nat Commun. 2012;3:1125. doi: 10.1038/ncomms2119. Nat Commun. 2012. PMID: 23047683 Free PMC article.
References
-
- Blount KF, Breaker RR. Nat Biotechnol. 2006;24:1558. - PubMed
-
- Calin GA, Croce CM. Oncogene. 2006;25:6202. - PubMed
-
- Poehlsgaard J, Douthwaite S. Nat Rev Microbiol. 2005;3:870. - PubMed
-
- Fedor MJ, Williamson JR. Nat Rev Mol Cell Biol. 2005;6:399. - PubMed
-
- Thomas JR, Hergenrother PJ. Chem Rev. 2008;108:1171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

