Validation of internal controls for extraction and amplification of nucleic acids from enteric viruses in water samples
- PMID: 21602369
- PMCID: PMC3127680
- DOI: 10.1128/AEM.00077-11
Validation of internal controls for extraction and amplification of nucleic acids from enteric viruses in water samples
Abstract
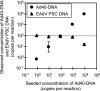
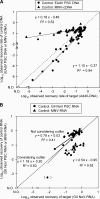
Inhibitors that reduce viral nucleic acid extraction efficiency and interfere with cDNA synthesis and/or polymerase activity affect the molecular detection of viruses in aquatic environments. To overcome these significant problems, we developed a methodology for assessing nucleic acid yields and DNA amplification efficiencies for environmental water samples. This involved adding particles of adenovirus type 5 and murine norovirus and newly developed primer-sharing controls, which are amplified with the same primer pairs and result in the same amplicon sizes as the targets, to these samples. We found that nucleic acid loss during the extraction process, rather than reverse transcription-PCR (RT-PCR) inhibition, more significantly attributed to underestimation of the presence of viral genomes in the environmental water samples tested in this study. Our success rate for satisfactorily amplifying viral RNAs and DNAs by RT-PCR was higher than that for obtaining adequate nucleic acid preparations. We found that inhibitory properties were greatest when we used larger sample volumes. A magnetic silica bead-based RNA extraction method effectively removed inhibitors that interfere with viral nucleic acid extraction and RT-PCR. To our knowledge, this is the first study to assess the inhibitory properties of environmental water samples by using both control virus particles and primer-sharing controls.
Figures

Similar articles
-
Extraction of viral nucleic acids: comparison of five automated nucleic acid extraction platforms.J Clin Virol. 2012 Jul;54(3):255-9. doi: 10.1016/j.jcv.2012.03.008. Epub 2012 Apr 13. J Clin Virol. 2012. PMID: 22503856
-
Presence of human immunodeficiency virus nucleic acids in wastewater and their detection by polymerase chain reaction.Appl Environ Microbiol. 1992 Dec;58(12):3984-90. doi: 10.1128/aem.58.12.3984-3990.1992. Appl Environ Microbiol. 1992. PMID: 1476440 Free PMC article.
-
Characterization of virus isolates by particle-associated nucleic acid PCR.J Clin Microbiol. 2005 Feb;43(2):716-20. doi: 10.1128/JCM.43.2.716-720.2005. J Clin Microbiol. 2005. PMID: 15695669 Free PMC article.
-
Environmental virology: from detection of virus in sewage and water by isolation to identification by molecular biology--a trip of over 50 years.Annu Rev Microbiol. 1995;49:461-87. doi: 10.1146/annurev.mi.49.100195.002333. Annu Rev Microbiol. 1995. PMID: 8561468 Review.
-
Sample preparation prior to molecular amplification: complexities and opportunities.Curr Opin Virol. 2014 Feb;4:66-70. doi: 10.1016/j.coviro.2013.12.004. Epub 2014 Jan 16. Curr Opin Virol. 2014. PMID: 24441295 Review.
Cited by
-
Comprehensive Study on Enteric Viruses and Indicators in Surface Water in Kyoto, Japan, During 2014-2015 Season.Food Environ Virol. 2018 Dec;10(4):353-364. doi: 10.1007/s12560-018-9355-3. Epub 2018 Aug 27. Food Environ Virol. 2018. PMID: 30151619
-
Viruses in wastewater: occurrence, abundance and detection methods.Sci Total Environ. 2020 Nov 25;745:140910. doi: 10.1016/j.scitotenv.2020.140910. Epub 2020 Jul 19. Sci Total Environ. 2020. PMID: 32758747 Free PMC article. Review.
-
Methodological approaches for monitoring opportunistic pathogens in premise plumbing: A review.Water Res. 2017 Jun 15;117:68-86. doi: 10.1016/j.watres.2017.03.046. Epub 2017 Mar 25. Water Res. 2017. PMID: 28390237 Free PMC article. Review.
-
Molecular diagnosis of COVID-19: Current situation and trend in China (Review).Exp Ther Med. 2020 Nov;20(5):13. doi: 10.3892/etm.2020.9142. Epub 2020 Aug 25. Exp Ther Med. 2020. PMID: 32934678 Free PMC article. Review.
-
Sewage surveillance for SARS-CoV-2: Molecular detection, quantification, and normalization factors.Curr Opin Environ Sci Health. 2022 Aug;28:100363. doi: 10.1016/j.coesh.2022.100363. Epub 2022 Apr 10. Curr Opin Environ Sci Health. 2022. PMID: 35694049 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

