Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1β activation and severe autoinflammation in mice
- PMID: 21600797
- PMCID: PMC3129608
- DOI: 10.1016/j.immuni.2011.02.020
Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1β activation and severe autoinflammation in mice
Abstract
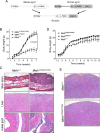
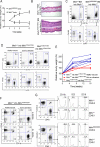
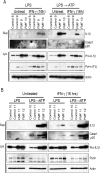
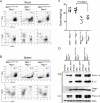
Missense mutations in the C-terminal B30.2 domain of pyrin cause familial Mediterranean fever (FMF), the most common Mendelian autoinflammatory disease. However, it remains controversial as to whether FMF is due to the loss of an inhibitor of inflammation or to the activity of a proinflammatory molecule. We generated both pyrin-deficient mice and "knockin" mice harboring mutant human B30.2 domains. Homozygous knockin, but not pyrin-deficient, mice exhibited spontaneous bone marrow-dependent inflammation similar to but more severe than human FMF. Caspase-1 was constitutively activated in knockin macrophages and active IL-1β was secreted when stimulated with lipopolysaccharide alone, which is also observed in FMF patients. The inflammatory phenotype of knockin mice was completely ablated by crossing with IL-1 receptor-deficient or adaptor molecule ASC-deficient mice, but not NLRP3-deficient mice. Thus, our data provide evidence for an ASC-dependent NLRP3-independent inflammasome in which gain-of-function pyrin mutations cause autoinflammatory disease.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



Comment in
-
A new twist on the PYRIN Mediterranean coast.Immunity. 2011 May 27;34(5):695-7. doi: 10.1016/j.immuni.2011.05.004. Immunity. 2011. PMID: 21616439
Similar articles
-
Dysregulated production of interleukin-1β upon activation of the NLRP3 inflammasome in patients with familial Mediterranean fever.Hum Immunol. 2015 Jul;76(7):488-95. doi: 10.1016/j.humimm.2015.06.007. Epub 2015 Jun 12. Hum Immunol. 2015. PMID: 26074413
-
Increased NLRP3-dependent interleukin 1β secretion in patients with familial Mediterranean fever: correlation with MEFV genotype.Ann Rheum Dis. 2014 Feb;73(2):462-9. doi: 10.1136/annrheumdis-2012-202774. Epub 2013 Mar 16. Ann Rheum Dis. 2014. PMID: 23505242
-
The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production.Proc Natl Acad Sci U S A. 2006 Jun 27;103(26):9982-7. doi: 10.1073/pnas.0602081103. Epub 2006 Jun 19. Proc Natl Acad Sci U S A. 2006. PMID: 16785446 Free PMC article.
-
Targeting IL-1beta in disease; the expanding role of NLRP3 inflammasome.Eur J Intern Med. 2010 Jun;21(3):157-63. doi: 10.1016/j.ejim.2010.03.005. Epub 2010 Mar 30. Eur J Intern Med. 2010. PMID: 20493414 Review.
-
Mechanisms of NLRP1-Mediated Autoinflammatory Disease in Humans and Mice.J Mol Biol. 2018 Jan 19;430(2):142-152. doi: 10.1016/j.jmb.2017.07.012. Epub 2017 Jul 19. J Mol Biol. 2018. PMID: 28733143 Review.
Cited by
-
Anakinra-Dependent Recurrent Pericarditis: The Role of the R202Q Variant of the MEFV Gene.J Clin Med. 2024 Oct 11;13(20):6051. doi: 10.3390/jcm13206051. J Clin Med. 2024. PMID: 39458001 Free PMC article.
-
Negative regulation of NLRP3 inflammasome signaling.Protein Cell. 2013 Apr;4(4):251-8. doi: 10.1007/s13238-013-2128-8. Epub 2013 Mar 21. Protein Cell. 2013. PMID: 23519777 Free PMC article. Review.
-
Regulated cell death in neutrophils: From apoptosis to NETosis and pyroptosis.Semin Immunol. 2023 Nov;70:101849. doi: 10.1016/j.smim.2023.101849. Epub 2023 Nov 6. Semin Immunol. 2023. PMID: 37939552 Free PMC article. Review.
-
Mediterranean fever gene variants modify clinical phenotypes of idiopathic multi-centric Castleman disease.Clin Exp Immunol. 2021 Oct;206(1):91-98. doi: 10.1111/cei.13632. Epub 2021 Jul 26. Clin Exp Immunol. 2021. PMID: 34096620 Free PMC article. Clinical Trial.
-
Cholesterol, Inflammasomes, and Atherogenesis.Curr Cardiovasc Risk Rep. 2012 Feb 1;6(1):45-52. doi: 10.1007/s12170-011-0212-2. Curr Cardiovasc Risk Rep. 2012. PMID: 22368729 Free PMC article.
References
-
- Belkhir R, Moulonguet-Doleris L, Hachulla E, Prinseau J, Baglin A, Hanslik T. Treatment of familial Mediterranean fever with anakinra. Ann. Intern. Med. 2007;146:825–826. - PubMed
-
- Bilginer Y, Ayaz NA, Ozen S. Anti-IL-1 treatment for secondary amyloidosis in an adolescent with FMF and Behcet's disease. Clin. Rheumatol. 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

