Beyond natural antimicrobial peptides: multimeric peptides and other peptidomimetic approaches
- PMID: 21598022
- PMCID: PMC11114707
- DOI: 10.1007/s00018-011-0717-3
Beyond natural antimicrobial peptides: multimeric peptides and other peptidomimetic approaches
Abstract
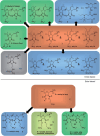
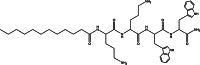
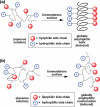
Naturally occurring antimicrobial peptides (AMPs) present several drawbacks that strongly limit their development into therapeutically valuable antibiotics. These include susceptibility to protease degradation and high costs of manufacture. To overcome these problems, researchers have tried to develop mimics or peptidomimetics endowed with better properties, while retaining the basic features of membrane-active natural AMPs such as cationic charge and amphipathic design. Protein epitope mimetics, multimeric (dendrimeric) peptides, oligoacyllysines, ceragenins, synthetic lipidated peptides, peptoids and other foldamers are some of the routes explored so far. The synthetic approach has led to compounds that have already entered clinical evaluation for the treatment of specific conditions, such as Staphylococcus (MRSA) infections. Should these trials be successful, an important proof-of-concept would be established, showing that synthetic oligomers rather than naturally occurring molecules could bring peptide-based antibiotics to clinical practice and the drug market for local and systemic treatment of medical conditions associated with multi-drug resistant pathogens.
Conflict of interest statement
One of the authors declares competing financial interests. Andrea Giuliani is an executive board member and minor shareholder of Spider Biotech S.r.l. (
Figures
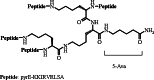
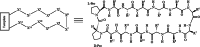
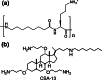
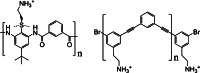
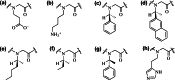
Similar articles
-
Norbornane-based cationic antimicrobial peptidomimetics targeting the bacterial membrane.Eur J Med Chem. 2018 Dec 5;160:9-22. doi: 10.1016/j.ejmech.2018.09.072. Epub 2018 Oct 3. Eur J Med Chem. 2018. PMID: 30316060 Free PMC article.
-
Antibacterial evaluation of structurally amphipathic, membrane active small cationic peptidomimetics: synthesized by incorporating 3-amino benzoic acid as peptidomimetic element.Eur J Med Chem. 2014 Aug 18;83:102-15. doi: 10.1016/j.ejmech.2014.06.023. Epub 2014 Jun 12. Eur J Med Chem. 2014. PMID: 24953028
-
Towards the Development of Synthetic Antibiotics: Designs Inspired by Natural Antimicrobial Peptides.Curr Med Chem. 2016;23(41):4610-4624. doi: 10.2174/0929867323666160825162435. Curr Med Chem. 2016. PMID: 27570165 Review.
-
Recent Advances in Amphipathic Peptidomimetics as Antimicrobial Agents to Combat Drug Resistance.Molecules. 2024 May 24;29(11):2492. doi: 10.3390/molecules29112492. Molecules. 2024. PMID: 38893366 Free PMC article. Review.
-
Design and synthesis of cell selective α/β-diastereomeric peptidomimetic with potent in vivo antibacterial activity against methicillin resistant S. Aureus.Bioorg Chem. 2018 Feb;76:538-547. doi: 10.1016/j.bioorg.2017.12.020. Epub 2017 Dec 26. Bioorg Chem. 2018. PMID: 29310083
Cited by
-
LAMP: A Database Linking Antimicrobial Peptides.PLoS One. 2013 Jun 18;8(6):e66557. doi: 10.1371/journal.pone.0066557. Print 2013. PLoS One. 2013. PMID: 23825543 Free PMC article.
-
Antimicrobial peptides and peptidomimetics - potent therapeutic allies for staphylococcal infections.Curr Pharm Des. 2015;21(16):2073-88. doi: 10.2174/1381612821666150310102702. Curr Pharm Des. 2015. PMID: 25760338 Free PMC article. Review.
-
S100 Proteins as Novel Therapeutic Targets in Psoriasis and Other Autoimmune Diseases.Molecules. 2022 Oct 6;27(19):6640. doi: 10.3390/molecules27196640. Molecules. 2022. PMID: 36235175 Free PMC article. Review.
-
Peptoid Library Agar Diffusion (PLAD) Assay for the High-Throughput Identification of Antimicrobial Peptoids.ACS Comb Sci. 2016 Jun 13;18(6):287-91. doi: 10.1021/acscombsci.6b00039. Epub 2016 May 26. ACS Comb Sci. 2016. PMID: 27186808 Free PMC article.
-
Antimicrobial peptides and their analogs: searching for new potential therapeutics.Perspect Medicin Chem. 2014 Oct 12;6:73-80. doi: 10.4137/PMC.S13215. eCollection 2014. Perspect Medicin Chem. 2014. PMID: 25374459 Free PMC article.
References
-
- Giuliani A, Rinaldi AC. Antimicrobial peptides. Methods and protocols. Methods in molecular biology. New York: Humana Press; 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

