Neuropeptides contribute to peripheral nociceptive sensitization by regulating interleukin-1β production in keratinocytes
- PMID: 21596883
- PMCID: PMC3123433
- DOI: 10.1213/ANE.0b013e31821a0258
Neuropeptides contribute to peripheral nociceptive sensitization by regulating interleukin-1β production in keratinocytes
Abstract
Background: It is increasingly evident that there is a close connection between the generation of cutaneous inflammatory cytokines and elevated neuropeptide signaling in complex regional pain syndrome (CRPS) patients. Previously, we observed in the rat tibia fracture model of CRPS that activation of caspase-1 containing NALP1 inflammasomes was required for interleukin (IL)-1β production in keratinocytes, and that administration of an IL-1 receptor antagonist (anakinra) reduced the fracture-induced hindpaw mechanical allodynia. We therefore hypothesized that neuropeptides lead to nociceptive sensitization through activation of the skin's innate immune system by enhancing inflammasome expression and caspase-1 activity.
Methods: We determined whether the neuropeptides substance P (SP) and calcitonin gene-related peptide (CGRP) require IL-1β to support nociceptive sensitization when injected into mouse hindpaw skin by testing mechanical allodynia. We then investigated whether these neuropeptides could stimulate production of IL-1β in a keratinocyte cell line (REKs), and could increase the expression of inflammasome component proteins including NALP1 and caspase-1. Finally, we determined whether neuropeptide-stimulated IL-1β production required activation of caspase-1 and cathepsin B.
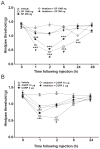
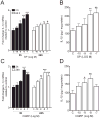
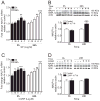
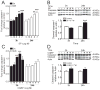
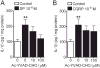
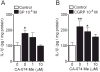
Results: Intraplantar injections of SP and CGRP lead to allodynia in mouse hindpaws but CGRP was approximately 10-fold less potent in causing this response. Moreover, systemic administration of the IL-1 receptor (IL-1R) antagonist anakinra prevented sensitization after neuropeptide injection. Also, mouse skin keratinocytes express IL-1R, which is up-regulated after local neuropeptide application. In vitro data demonstrated that both SP and CGRP increased IL-1β gene and protein expression in REKs in a dose-dependent manner. Furthermore, SP time- and dose-dependently up-regulated NALP1 and caspase-1 mRNA and protein levels in REKs. In contrast, CGRP time- and dose-dependently enhanced NALP1 and caspase-1 mRNA levels without causing a significant change in NALP1 or caspase-1 protein expression in REKs. Inhibition of caspase-1 activity using the selective inhibitor Ac-YVAD-CHO reduced SP and, less effectively, CGRP induced increases in IL-1β production in REK cells. The selective cathepsin B inhibitor CA-74Me inhibited neuropeptide induced IL-1β production in REKs as well.
Conclusions: Collectively, these results demonstrate that neuropeptides induce nociceptive sensitization by enhancing IL-1 β production in keratinocytes. Neuropeptides rely on both caspase-1 and cathepsin B for this enhanced production. Neurocutaneous signaling involving neuropeptide activation of the innate immunity may contribute to pain in CRPS patients.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
The Role of Neuroinflammation in Complex Regional Pain Syndrome: A Comprehensive Review.J Pain Res. 2023 Sep 6;16:3061-3073. doi: 10.2147/JPR.S423733. eCollection 2023. J Pain Res. 2023. PMID: 37701560 Free PMC article. Review.
-
The NALP1 inflammasome controls cytokine production and nociception in a rat fracture model of complex regional pain syndrome.Pain. 2009 Dec 15;147(1-3):277-86. doi: 10.1016/j.pain.2009.09.032. Epub 2009 Oct 22. Pain. 2009. PMID: 19853379 Free PMC article.
-
The role of enhanced cutaneous IL-1beta signaling in a rat tibia fracture model of complex regional pain syndrome.Pain. 2009 Aug;144(3):303-313. doi: 10.1016/j.pain.2009.04.033. Epub 2009 May 26. Pain. 2009. PMID: 19473768 Free PMC article.
-
Fracture induces keratinocyte activation, proliferation, and expression of pro-nociceptive inflammatory mediators.Pain. 2010 Dec;151(3):843-852. doi: 10.1016/j.pain.2010.09.026. Epub 2010 Oct 12. Pain. 2010. PMID: 20934254 Free PMC article.
-
The Rodent Tibia Fracture Model: A Critical Review and Comparison With the Complex Regional Pain Syndrome Literature.J Pain. 2018 Oct;19(10):1102.e1-1102.e19. doi: 10.1016/j.jpain.2018.03.018. Epub 2018 Apr 21. J Pain. 2018. PMID: 29684510 Free PMC article. Review.
Cited by
-
Epithelial-Neuronal Communication in the Colon: Implications for Visceral Pain.Trends Neurosci. 2020 Mar;43(3):170-181. doi: 10.1016/j.tins.2019.12.007. Epub 2020 Jan 23. Trends Neurosci. 2020. PMID: 31983457 Free PMC article. Review.
-
Mechanosensory and ATP Release Deficits following Keratin14-Cre-Mediated TRPA1 Deletion Despite Absence of TRPA1 in Murine Keratinocytes.PLoS One. 2016 Mar 15;11(3):e0151602. doi: 10.1371/journal.pone.0151602. eCollection 2016. PLoS One. 2016. PMID: 26978657 Free PMC article.
-
Potential Nociceptive Role of the Thoracolumbar Fascia: A Scope Review Involving In Vivo and Ex Vivo Studies.J Clin Med. 2021 Sep 24;10(19):4342. doi: 10.3390/jcm10194342. J Clin Med. 2021. PMID: 34640360 Free PMC article. Review.
-
The Role of Neuroinflammation in Complex Regional Pain Syndrome: A Comprehensive Review.J Pain Res. 2023 Sep 6;16:3061-3073. doi: 10.2147/JPR.S423733. eCollection 2023. J Pain Res. 2023. PMID: 37701560 Free PMC article. Review.
-
Acute and chronic phases of complex regional pain syndrome in mice are accompanied by distinct transcriptional changes in the spinal cord.Mol Pain. 2013 Aug 8;9:40. doi: 10.1186/1744-8069-9-40. Mol Pain. 2013. PMID: 23924076 Free PMC article.
References
-
- Birklein F, Schmelz M. Neuropeptides, neurogenic inflammation and complex regional pain syndrome (CRPS) Neurosci Lett. 2008;437:199–202. - PubMed
-
- Schinkel C, Gaertner A, Zaspel J, Zedler S, Faist E, Schuermann M. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. Clin J Pain. 2006;22:235–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

