Regulation of type 17 helper T-cell function by nitric oxide during inflammation
- PMID: 21576463
- PMCID: PMC3107290
- DOI: 10.1073/pnas.1100667108
Regulation of type 17 helper T-cell function by nitric oxide during inflammation
Abstract
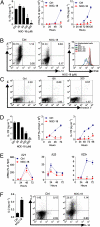
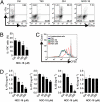
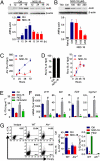
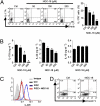
Type 17 helper T (Th17) cells are implicated in the pathogenesis many of human autoimmune diseases. Development of Th17 can be enhanced by the activation of aryl hydrocarbon receptor (AHR) whose ligands include the environmental pollutant dioxin, potentially linking environmental factors to the increased prevalence of autoimmune disease. We report here that nitric oxide (NO) can suppress the proliferation and function of polarized murine and human Th17 cells. NO also inhibits AHR expression in Th17 cells and the downstream events of AHR activation, including IL-22, IL-23 receptor, and Cyp1a1. Conversely, NO did not affect the polarization of Th17 cells from mice deficient in AHR. Furthermore, mice lacking inducible nitric oxide synthase (Nos2(-/-)) developed more severe experimental autoimmune encephalomyelitis than WT mice, with elevated AHR expression, increased IL-17A, and IL-22 synthesis. NO may therefore represent an important endogenous regulator to prevent overexpansion of Th17 cells and control of autoimmune diseases caused by environmental pollutants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells.J Exp Med. 2009 Jan 16;206(1):43-9. doi: 10.1084/jem.20081438. Epub 2008 Dec 29. J Exp Med. 2009. PMID: 19114668 Free PMC article.
-
Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells.Eur J Immunol. 2010 Sep;40(9):2450-9. doi: 10.1002/eji.201040461. Eur J Immunol. 2010. PMID: 20706985
-
The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins.Nature. 2008 May 1;453(7191):106-9. doi: 10.1038/nature06881. Epub 2008 Mar 23. Nature. 2008. PMID: 18362914
-
Inducible NO synthase and antibacterial host defence in times of Th17/Th22/T22 immunity.Cell Microbiol. 2011 Mar;13(3):340-8. doi: 10.1111/j.1462-5822.2010.01559.x. Epub 2010 Dec 28. Cell Microbiol. 2011. PMID: 21199257 Review.
-
Th17 cells in inflammation.Int Immunopharmacol. 2011 Mar;11(3):319-22. doi: 10.1016/j.intimp.2010.10.004. Epub 2010 Oct 28. Int Immunopharmacol. 2011. PMID: 21035432 Review.
Cited by
-
Leishmania guyanensis suppressed inducible nitric oxide synthase provoked by its viral endosymbiont.Front Cell Infect Microbiol. 2022 Aug 12;12:944819. doi: 10.3389/fcimb.2022.944819. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36034693 Free PMC article.
-
Nitric oxide inhibits the accumulation of CD4+CD44hiTbet+CD69lo T cells in mycobacterial infection.Eur J Immunol. 2012 Dec;42(12):3267-79. doi: 10.1002/eji.201142158. Epub 2012 Sep 26. Eur J Immunol. 2012. PMID: 22890814 Free PMC article.
-
Myeloid cell-derived inducible nitric oxide synthase suppresses M1 macrophage polarization.Nat Commun. 2015 Mar 27;6:6676. doi: 10.1038/ncomms7676. Nat Commun. 2015. PMID: 25813085 Free PMC article.
-
Toward understanding the role of aryl hydrocarbon receptor in the immune system: current progress and future trends.Biomed Res Int. 2014;2014:520763. doi: 10.1155/2014/520763. Epub 2014 Jan 6. Biomed Res Int. 2014. PMID: 24527450 Free PMC article. Review.
-
ATP Triggers Human Th9 Cell Differentiation via Nitric Oxide-Mediated mTOR-HIF1α Pathway.Front Immunol. 2019 May 20;10:1120. doi: 10.3389/fimmu.2019.01120. eCollection 2019. Front Immunol. 2019. PMID: 31164892 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

