Telomere dysfunction and chromosome instability
- PMID: 21575645
- PMCID: PMC3178001
- DOI: 10.1016/j.mrfmmm.2011.04.008
Telomere dysfunction and chromosome instability
Abstract
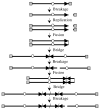
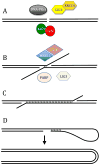
The ends of chromosomes are composed of a short repeat sequence and associated proteins that together form a cap, called a telomere, that keeps the ends from appearing as double-strand breaks (DSBs) and prevents chromosome fusion. The loss of telomeric repeat sequences or deficiencies in telomeric proteins can result in chromosome fusion and lead to chromosome instability. The similarity between chromosome rearrangements resulting from telomere loss and those found in cancer cells implicates telomere loss as an important mechanism for the chromosome instability contributing to human cancer. Telomere loss in cancer cells can occur through gradual shortening due to insufficient telomerase, the protein that maintains telomeres. However, cancer cells often have a high rate of spontaneous telomere loss despite the expression of telomerase, which has been proposed to result from a combination of oncogene-mediated replication stress and a deficiency in DSB repair in telomeric regions. Chromosome fusion in mammalian cells primarily involves nonhomologous end joining (NHEJ), which is the major form of DSB repair. Chromosome fusion initiates chromosome instability involving breakage-fusion-bridge (B/F/B) cycles, in which dicentric chromosomes form bridges and break as the cell attempts to divide, repeating the process in subsequent cell cycles. Fusion between sister chromatids results in large inverted repeats on the end of the chromosome, which amplify further following additional B/F/B cycles. B/F/B cycles continue until the chromosome acquires a new telomere, most often by translocation of the end of another chromosome. The instability is not confined to a chromosome that loses its telomere, because the instability is transferred to the chromosome donating a translocation. Moreover, the amplified regions are unstable and form extrachromosomal DNA that can reintegrate at new locations. Knowledge concerning the factors promoting telomere loss and its consequences is therefore important for understanding chromosome instability in human cancer.
Copyright © 2011 Elsevier B.V. All rights reserved.
Conflict of interest statement
No conflicts of interest to disclose.
Figures
Similar articles
-
Telomeres and chromosome instability.DNA Repair (Amst). 2006 Sep 8;5(9-10):1082-92. doi: 10.1016/j.dnarep.2006.05.030. Epub 2006 Jun 19. DNA Repair (Amst). 2006. PMID: 16784900 Review.
-
Mechanisms of telomere loss and their consequences for chromosome instability.Front Oncol. 2012 Oct 4;2:135. doi: 10.3389/fonc.2012.00135. eCollection 2012. Front Oncol. 2012. PMID: 23061048 Free PMC article.
-
The role of ATM in the deficiency in nonhomologous end-joining near telomeres in a human cancer cell line.PLoS Genet. 2013 Mar;9(3):e1003386. doi: 10.1371/journal.pgen.1003386. Epub 2013 Mar 28. PLoS Genet. 2013. PMID: 23555296 Free PMC article.
-
Chromosome instability as a result of double-strand breaks near telomeres in mouse embryonic stem cells.Mol Cell Biol. 2002 Jul;22(13):4836-50. doi: 10.1128/MCB.22.13.4836-4850.2002. Mol Cell Biol. 2002. PMID: 12052890 Free PMC article.
-
Telomeres, chromosome instability and cancer.Nucleic Acids Res. 2006 May 8;34(8):2408-17. doi: 10.1093/nar/gkl303. Print 2006. Nucleic Acids Res. 2006. PMID: 16682448 Free PMC article. Review.
Cited by
-
Telomeres and viruses: common themes of genome maintenance.Front Oncol. 2012 Dec 31;2:201. doi: 10.3389/fonc.2012.00201. eCollection 2012. Front Oncol. 2012. PMID: 23293769 Free PMC article.
-
Origin of cardiomyocytes in the adult heart.Circ Res. 2015 Jan 2;116(1):150-66. doi: 10.1161/CIRCRESAHA.116.303595. Circ Res. 2015. PMID: 25552694 Free PMC article. Review.
-
Homology-mediated end-capping as a primary step of sister chromatid fusion in the breakage-fusion-bridge cycles.Nucleic Acids Res. 2013 Nov;41(21):9732-40. doi: 10.1093/nar/gkt762. Epub 2013 Aug 23. Nucleic Acids Res. 2013. PMID: 23975201 Free PMC article.
-
Base excision repair: a critical player in many games.DNA Repair (Amst). 2014 Jul;19:14-26. doi: 10.1016/j.dnarep.2014.03.030. Epub 2014 Apr 26. DNA Repair (Amst). 2014. PMID: 24780558 Free PMC article. Review.
-
Differences in the recruitment of DNA repair proteins at subtelomeric and interstitial I-SceI endonuclease-induced DNA double-strand breaks.DNA Repair (Amst). 2017 Jan;49:1-8. doi: 10.1016/j.dnarep.2016.10.008. Epub 2016 Nov 5. DNA Repair (Amst). 2017. PMID: 27842255 Free PMC article.
References
-
- Muller HJ. The remaking of chromosomes. The collecting net-Woods Hole. 1938;13:181–198.
-
- Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12:1133–1138. - PubMed
-
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. - PubMed
-
- d’ Adda di Fagagna FdAd, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

