Defective sumoylation pathway directs congenital heart disease
- PMID: 21563299
- PMCID: PMC5031480
- DOI: 10.1002/bdra.20816
Defective sumoylation pathway directs congenital heart disease
Abstract
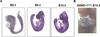
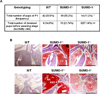
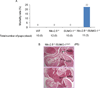
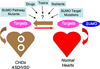
Congenital heart defects (CHDs) are the most common of all birth defects, yet molecular mechanism(s) underlying highly prevalent atrial septal defects (ASDs) and ventricular septal defects (VSDs) have remained elusive. We demonstrate the indispensability of "balanced" posttranslational small ubiquitin-like modifier (SUMO) conjugation-deconjugation pathway for normal cardiac development. Both hetero- and homozygous SUMO-1 knockout mice exhibited ASDs and VSDs with high mortality rates, which were rescued by cardiac reexpression of the SUMO-1 transgene. Because SUMO-1 was also involved in cleft lip/palate in human patients, the previous findings provided a powerful rationale to question whether SUMO-1 was mutated in infants born with cleft palates and ASDs. Sequence analysis of DNA from newborn screening blood spots revealed a single 16 bp substitution in the SUMO-1 regulatory promoter of a patient displaying both oral-facial clefts and ASDs. Diminished sumoylation activity whether by genetics, environmental toxins, and/or pharmaceuticals may significantly contribute to susceptibility to the induction of congenital heart disease worldwide. Birth Defects Research (Part A) 2011. © 2011 Wiley-Liss, Inc.
Copyright © 2011 Wiley-Liss, Inc.
Figures


Similar articles
-
Enhanced desumoylation in murine hearts by overexpressed SENP2 leads to congenital heart defects and cardiac dysfunction.J Mol Cell Cardiol. 2012 Mar;52(3):638-49. doi: 10.1016/j.yjmcc.2011.11.011. Epub 2011 Dec 1. J Mol Cell Cardiol. 2012. PMID: 22155005 Free PMC article.
-
Sumoylation in Craniofacial Disorders.Adv Exp Med Biol. 2017;963:323-335. doi: 10.1007/978-3-319-50044-7_19. Adv Exp Med Biol. 2017. PMID: 28197921 Review.
-
FGF signalling and SUMO modification: new players in the aetiology of cleft lip and/or palate.Trends Genet. 2007 Dec;23(12):631-40. doi: 10.1016/j.tig.2007.09.002. Epub 2007 Nov 5. Trends Genet. 2007. PMID: 17981355 Review.
-
TBX22 missense mutations found in patients with X-linked cleft palate affect DNA binding, sumoylation, and transcriptional repression.Am J Hum Genet. 2007 Oct;81(4):700-12. doi: 10.1086/521033. Epub 2007 Aug 16. Am J Hum Genet. 2007. PMID: 17846996 Free PMC article.
-
SUMO1 haploinsufficiency leads to cleft lip and palate.Science. 2006 Sep 22;313(5794):1751. doi: 10.1126/science.1128406. Science. 2006. PMID: 16990542
Cited by
-
Function and regulation of ubiquitin-like SUMO system in heart.Front Cell Dev Biol. 2023 Nov 16;11:1294717. doi: 10.3389/fcell.2023.1294717. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38033852 Free PMC article. Review.
-
SUMO: From Bench to Bedside.Physiol Rev. 2020 Oct 1;100(4):1599-1619. doi: 10.1152/physrev.00025.2019. Physiol Rev. 2020. PMID: 32666886 Free PMC article. Review.
-
Role of Posttranslational Modifications of Proteins in Cardiovascular Disease.Oxid Med Cell Longev. 2022 Jul 9;2022:3137329. doi: 10.1155/2022/3137329. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35855865 Free PMC article. Review.
-
GPS-SUMO: a tool for the prediction of sumoylation sites and SUMO-interaction motifs.Nucleic Acids Res. 2014 Jul;42(Web Server issue):W325-30. doi: 10.1093/nar/gku383. Epub 2014 May 31. Nucleic Acids Res. 2014. PMID: 24880689 Free PMC article.
-
Roles of ubiquitination and SUMOylation on prostate cancer: mechanisms and clinical implications.Int J Mol Sci. 2015 Feb 27;16(3):4560-80. doi: 10.3390/ijms16034560. Int J Mol Sci. 2015. PMID: 25734985 Free PMC article. Review.
References
-
- Alkuraya FS, Saadi I, Lund JJ, Turbe-Doan A, Morton CC, Maas RL. SUMO1 haploinsufficiency leads to cleft lip and palate. Science. 2006;313:1751. - PubMed
-
- Arnaoutov A, Azuma Y, Ribbeck K, Joseph J, Boyarchuk Y, Karpova T, McNally J, Dasso M. Crm1 is a mitotic effector of Ran-GTP in somatic cells. Nat Cell Biol. 2005;7:626–632. - PubMed
-
- Baldini A. Dissecting contiguous gene defects: TBX1. Curr Opin Genet Dev. 2005;15:279–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

