Comparison of analytical platforms for cerebrospinal fluid measures of β-amyloid 1-42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology
- PMID: 21555603
- PMCID: PMC3154969
- DOI: 10.1001/archneurol.2011.105
Comparison of analytical platforms for cerebrospinal fluid measures of β-amyloid 1-42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology
Abstract
Background: Cerebrospinal fluid (CSF) biomarkers of Alzheimer disease (AD) are currently being considered for inclusion in revised diagnostic criteria for research and/or clinical purposes to increase the certainty of antemortem diagnosis.
Objective: To test whether CSF biomarker assays differ in their ability to identify true markers of underlying AD pathology (eg, amyloid plaques and/or neurofibrillary tangles) in living individuals.
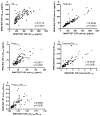
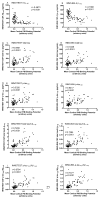
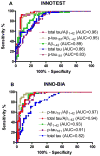
Design: We compared the performances of the 2 most commonly used platforms, INNOTEST enzyme-linked immunosorbent assay and INNO-BIA AlzBio3, for measurement of CSF β-amyloid (Aβ) and tau proteins to identify the presence of amyloid plaques in a research cohort (n=103). Values obtained for CSF Aβ1-42, total tau, and phosphorylated tau 181 (p-tau(181)) using the 2 assay platforms were compared with brain amyloid load as assessed by positron emission tomography using the amyloid imaging agent Pittsburgh compound B.
Setting: The Knight Alzheimer's Disease Research Center at Washington University in St Louis, Missouri.
Subjects: Research volunteers who were cognitively normal or had mild to moderate AD dementia.
Results: The 2 assay platforms yielded different (approximately 2- to 6-fold) absolute values for the various analytes, but relative values were highly correlated. The CSF Aβ1-42 correlated inversely and tau and p-tau(181) correlated positively with the amount of cortical Pittsburgh compound B binding, albeit to differing degrees. Both assays yielded similar patterns of CSF biomarker correlations with amyloid load. The ratios of total tau to Aβ1-42 and p-tau(181) to Aβ1-42 outperformed any single analyte, including Aβ1-42, in discriminating individuals with vs without cortical amyloid.
Conclusions: The INNOTEST and INNO-BIA CSF platforms perform equally well in identifying individuals with underlying amyloid plaque pathology. Differences in absolute values, however, point to the need for assay-specific diagnostic cutoff values.
Figures
Similar articles
-
Comparison of cerebrospinal fluid levels of tau and Aβ 1-42 in Alzheimer disease and frontotemporal degeneration using 2 analytical platforms.Arch Neurol. 2012 Aug;69(8):1018-25. doi: 10.1001/archneurol.2012.26. Arch Neurol. 2012. PMID: 22490326 Free PMC article.
-
Comparison of two analytical platforms for the clinical qualification of Alzheimer's disease biomarkers in pathologically-confirmed dementia.J Alzheimers Dis. 2013;33(1):117-31. doi: 10.3233/JAD-2012-121246. J Alzheimers Dis. 2013. PMID: 22936010 Clinical Trial.
-
Comparison of Two Analytical Platforms in Cerebrospinal Fluid Biomarkers for the Classification of Alzheimer's Disease Spectrum with Amyloid PET Imaging.J Alzheimers Dis. 2020;75(3):949-958. doi: 10.3233/JAD-191331. J Alzheimers Dis. 2020. PMID: 32390627
-
Simultaneous analysis of cerebrospinal fluid biomarkers using microsphere-based xMAP multiplex technology for early detection of Alzheimer's disease.Methods. 2012 Apr;56(4):484-93. doi: 10.1016/j.ymeth.2012.03.023. Epub 2012 Apr 6. Methods. 2012. PMID: 22503777 Review.
-
Clinical utility and analytical challenges in measurement of cerebrospinal fluid amyloid-β(1-42) and τ proteins as Alzheimer disease biomarkers.Clin Chem. 2013 Jun;59(6):903-16. doi: 10.1373/clinchem.2013.202937. Epub 2013 Mar 21. Clin Chem. 2013. PMID: 23519967 Free PMC article. Review.
Cited by
-
Comparison of cerebrospinal fluid levels of tau and Aβ 1-42 in Alzheimer disease and frontotemporal degeneration using 2 analytical platforms.Arch Neurol. 2012 Aug;69(8):1018-25. doi: 10.1001/archneurol.2012.26. Arch Neurol. 2012. PMID: 22490326 Free PMC article.
-
Early onset diagnosis in Alzheimer's disease patients via amyloid-β oligomers-sensing probe in cerebrospinal fluid.Nat Commun. 2024 Feb 2;15(1):1004. doi: 10.1038/s41467-024-44818-x. Nat Commun. 2024. PMID: 38307843 Free PMC article.
-
Acute sleep loss decreases CSF-to-blood clearance of Alzheimer's disease biomarkers.Alzheimers Dement. 2023 Jul;19(7):3055-3064. doi: 10.1002/alz.12930. Epub 2023 Jan 25. Alzheimers Dement. 2023. PMID: 36695437 Free PMC article.
-
Longitudinal Basal Forebrain Degeneration Interacts with TREM2/C3 Biomarkers of Inflammation in Presymptomatic Alzheimer's Disease.J Neurosci. 2020 Feb 26;40(9):1931-1942. doi: 10.1523/JNEUROSCI.1184-19.2019. Epub 2020 Jan 8. J Neurosci. 2020. PMID: 31915256 Free PMC article.
-
Neurofilament Proteins as Biomarkers to Monitor Neurological Diseases and the Efficacy of Therapies.Front Neurosci. 2021 Sep 27;15:689938. doi: 10.3389/fnins.2021.689938. eCollection 2021. Front Neurosci. 2021. PMID: 34646114 Free PMC article. Review.
References
-
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. - PubMed
-
- Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in “normal” aging: Evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1174. - PubMed
-
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. - PubMed
-
- Knopman D, DeKosky S, Cummings J, et al. Practice parameter: Diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143–1153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG026276-01/AG/NIA NIH HHS/United States
- AG10124/AG/NIA NIH HHS/United States
- 1RC AG036535/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- RC2 AG036535/AG/NIA NIH HHS/United States
- P30 NS057105-01/NS/NINDS NIH HHS/United States
- P50 AG005681-18/AG/NIA NIH HHS/United States
- P30 NS048056/NS/NINDS NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- UL1 RR024992-05/RR/NCRR NIH HHS/United States
- P30 AG010124-11/AG/NIA NIH HHS/United States
- P30 NS057105/NS/NINDS NIH HHS/United States
- P50 AG05681/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- U01 AG024904-04/AG/NIA NIH HHS/United States
- P01 AG003991-16/AG/NIA NIH HHS/United States
- RC2 AG036535-02/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P01 AG03991/AG/NIA NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- U19 AG032438/AG/NIA NIH HHS/United States
- P30 NS048056-06/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

