Extended safety and efficacy studies of a live attenuated double leucine and pantothenate auxotroph of Mycobacterium tuberculosis as a vaccine candidate
- PMID: 21549795
- PMCID: PMC3146342
- DOI: 10.1016/j.vaccine.2011.04.066
Extended safety and efficacy studies of a live attenuated double leucine and pantothenate auxotroph of Mycobacterium tuberculosis as a vaccine candidate
Abstract
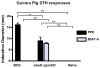
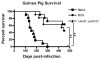
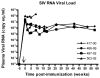
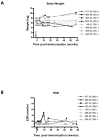
We have previously described the development of a live, fully attenuated Mycobacterium tuberculosis (Mtb) vaccine candidate strain with two independent attenuating auxotrophic mutations in leucine and pantothenate biosynthesis. In the present work, those studies have been extended to include testing for protective efficacy in a long-term guinea pig survival model and safety testing in the highly tuberculosis susceptible Rhesus macaque. To model the safety of the ΔleuD ΔpanCD strain in HIV-infected human populations, a Simian immunodeficiency virus (SIV)-infected Rhesus macaque group was included. Immunization with the non-replicating ΔleuD ΔpanCD conferred long-term protection against challenge with virulent M. tuberculosis equivalent to that afforded by BCG as measured by guinea pig survival. In safety studies, clinical, hematological and bacteriological monitoring of both SIV-positive and SIV-negative Rhesus macaques immunized with ΔleuD ΔpanCD, revealed no vaccine-associated adverse effects. The results support the further development of the ΔleuD ΔpanCD strain as a viable tuberculosis (TB) vaccine candidate.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Protection elicited by a double leucine and pantothenate auxotroph of Mycobacterium tuberculosis in guinea pigs.Infect Immun. 2004 May;72(5):3031-7. doi: 10.1128/IAI.72.5.3031-3037.2004. Infect Immun. 2004. PMID: 15102816 Free PMC article.
-
A recombinant attenuated Mycobacterium tuberculosis vaccine strain is safe in immunosuppressed simian immunodeficiency virus-infected infant macaques.Clin Vaccine Immunol. 2012 Aug;19(8):1170-81. doi: 10.1128/CVI.00184-12. Epub 2012 Jun 13. Clin Vaccine Immunol. 2012. PMID: 22695156 Free PMC article.
-
Efficacy and safety of live attenuated persistent and rapidly cleared Mycobacterium tuberculosis vaccine candidates in non-human primates.Vaccine. 2009 Jul 23;27(34):4709-17. doi: 10.1016/j.vaccine.2009.05.050. Epub 2009 Jun 2. Vaccine. 2009. PMID: 19500524 Free PMC article.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6.J Intern Med. 2009 Jan;265(1):67-77. doi: 10.1111/j.1365-2796.2008.02051.x. J Intern Med. 2009. PMID: 19093961 Free PMC article. Review.
Cited by
-
Balancing Trained Immunity with Persistent Immune Activation and the Risk of Simian Immunodeficiency Virus Infection in Infant Macaques Vaccinated with Attenuated Mycobacterium tuberculosis or Mycobacterium bovis BCG Vaccine.Clin Vaccine Immunol. 2017 Jan 5;24(1):e00360-16. doi: 10.1128/CVI.00360-16. Print 2017 Jan. Clin Vaccine Immunol. 2017. PMID: 27655885 Free PMC article.
-
Mycobacterium tuberculosis Metabolism.Microbiol Spectr. 2019 Jul;7(4):10.1128/microbiolspec.gpp3-0067-2019. doi: 10.1128/microbiolspec.GPP3-0067-2019. Microbiol Spectr. 2019. PMID: 31350832 Free PMC article. Review.
-
Total Synthesis of the Antimycobacterial Natural Product Chlorflavonin and Analogs via a Late-Stage Ruthenium(II)-Catalyzed ortho-C(sp2)-H-Hydroxylation.Pharmaceuticals (Basel). 2022 Aug 10;15(8):984. doi: 10.3390/ph15080984. Pharmaceuticals (Basel). 2022. PMID: 36015133 Free PMC article.
-
Novel GMO-Based Vaccines against Tuberculosis: State of the Art and Biosafety Considerations.Vaccines (Basel). 2014 Jun 16;2(2):463-99. doi: 10.3390/vaccines2020463. Vaccines (Basel). 2014. PMID: 26344627 Free PMC article. Review.
-
Gene Transfer in Mycobacterium tuberculosis: Shuttle Phasmids to Enlightenment.Microbiol Spectr. 2014 Apr;2(2):10.1128/microbiolspec.MGM2-0037-2013. doi: 10.1128/microbiolspec.MGM2-0037-2013. Microbiol Spectr. 2014. PMID: 26105819 Free PMC article. Review.
References
-
- Dye C, Williams BG. The Population Dynamics and Control of Tuberculosis. Science. 2010;328(5980):856–61. - PubMed
-
- World Health Organization. Global Tuberculosis Control: A Short Update To The 2009 Report. World Health Organization; Geneva: 2009.
-
- Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006 Apr 8;367(9517):1173–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

