Ion channels, phosphorylation and mammalian sperm capacitation
- PMID: 21540868
- PMCID: PMC3739340
- DOI: 10.1038/aja.2010.69
Ion channels, phosphorylation and mammalian sperm capacitation
Abstract
Sexually reproducing animals require an orchestrated communication between spermatozoa and the egg to generate a new individual. Capacitation, a maturational complex phenomenon that occurs in the female reproductive tract, renders spermatozoa capable of binding and fusing with the oocyte, and it is a requirement for mammalian fertilization. Capacitation encompasses plasma membrane reorganization, ion permeability regulation, cholesterol loss and changes in the phosphorylation state of many proteins. Novel tools to study sperm ion channels, image intracellular ionic changes and proteins with better spatial and temporal resolution, are unraveling how modifications in sperm ion transport and phosphorylation states lead to capacitation. Recent evidence indicates that two parallel pathways regulate phosphorylation events leading to capacitation, one of them requiring activation of protein kinase A and the second one involving inactivation of ser/thr phosphatases. This review examines the involvement of ion transporters and phosphorylation signaling processes needed for spermatozoa to achieve capacitation. Understanding the molecular mechanisms leading to fertilization is central for societies to deal with rising male infertility rates, to develop safe male gamete-based contraceptives and to preserve biodiversity through better assisted fertilization strategies.
Figures
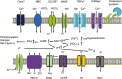
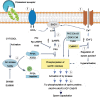
 Influx of HCO3− and Ca2+ stimulates an SACY.
Influx of HCO3− and Ca2+ stimulates an SACY.  Increasing intracellular cAMP concentrations.
Increasing intracellular cAMP concentrations.  High cAMP levels activate PKA. This activation can be blocked in vitro by addition of the inhibitors H-89, Rp-cAMPs and the peptide PKI.
High cAMP levels activate PKA. This activation can be blocked in vitro by addition of the inhibitors H-89, Rp-cAMPs and the peptide PKI.  In vivo, phosphorylation of PKA substrates is subjected to regulation by a ser/thr phosphatase, likely PP2A. This phosphatase is in turn downregulated by a member of the Src kinase family by still unknown mechanisms. Activation of Src kinase family members can be blocked by in vitro addition of the inhibitors SU6656 or SKI-606 (Bosutinib). The onset of PKA substrates phosphorylation is followed by activation of unidentified tyrosine kinases
In vivo, phosphorylation of PKA substrates is subjected to regulation by a ser/thr phosphatase, likely PP2A. This phosphatase is in turn downregulated by a member of the Src kinase family by still unknown mechanisms. Activation of Src kinase family members can be blocked by in vitro addition of the inhibitors SU6656 or SKI-606 (Bosutinib). The onset of PKA substrates phosphorylation is followed by activation of unidentified tyrosine kinases  and the promotion of tyrosine phosphorylation of sperm proteins
and the promotion of tyrosine phosphorylation of sperm proteins  (e.g., AKAP4, AKAP3, VCP and CABYR).
(e.g., AKAP4, AKAP3, VCP and CABYR).  Intracellular Ca2+ increase might affect the axoneme directly, through selective regulation of dyneins. In addition, Ca2+ upregulates CaMKIV, leading to hyperactivation. PKA, protein kinase A; PKC, protein kinase C; PKI, protein kinase inhibitor; SACY, soluble adenylyl cyclase; VCP, valosin-containing protein.
Intracellular Ca2+ increase might affect the axoneme directly, through selective regulation of dyneins. In addition, Ca2+ upregulates CaMKIV, leading to hyperactivation. PKA, protein kinase A; PKC, protein kinase C; PKI, protein kinase inhibitor; SACY, soluble adenylyl cyclase; VCP, valosin-containing protein.Similar articles
-
CFTR/ENaC-dependent regulation of membrane potential during human sperm capacitation is initiated by bicarbonate uptake through NBC.J Biol Chem. 2018 Jun 22;293(25):9924-9936. doi: 10.1074/jbc.RA118.003166. Epub 2018 May 9. J Biol Chem. 2018. PMID: 29743243 Free PMC article.
-
Functional human sperm capacitation requires both bicarbonate-dependent PKA activation and down-regulation of Ser/Thr phosphatases by Src family kinases.Mol Hum Reprod. 2013 Sep;19(9):570-80. doi: 10.1093/molehr/gat033. Epub 2013 Apr 29. Mol Hum Reprod. 2013. PMID: 23630234 Free PMC article.
-
Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors.J Biol Chem. 2010 Mar 12;285(11):7977-85. doi: 10.1074/jbc.M109.085845. Epub 2010 Jan 12. J Biol Chem. 2010. PMID: 20068039 Free PMC article.
-
Sperm Capacitation and Acrosome Reaction in Mammalian Sperm.Adv Anat Embryol Cell Biol. 2016;220:93-106. doi: 10.1007/978-3-319-30567-7_5. Adv Anat Embryol Cell Biol. 2016. PMID: 27194351 Review.
-
Signalling Events and Associated Pathways Related to the Mammalian Sperm Capacitation.Reprod Domest Anim. 2015 Oct;50(5):705-11. doi: 10.1111/rda.12541. Epub 2015 Aug 21. Reprod Domest Anim. 2015. PMID: 26294224 Review.
Cited by
-
pH Homeodynamics and Male Fertility: A Coordinated Regulation of Acid-Based Balance during Sperm Journey to Fertilization.Biomolecules. 2024 Jun 12;14(6):685. doi: 10.3390/biom14060685. Biomolecules. 2024. PMID: 38927088 Free PMC article. Review.
-
HDL mediates reverse cholesterol transport from ram spermatozoa and induces hyperactivated motility.Biol Reprod. 2021 Jun 4;104(6):1271-1281. doi: 10.1093/biolre/ioab035. Biol Reprod. 2021. PMID: 33674849 Free PMC article.
-
Proteomic Changes in Human Sperm During Sequential in vitro Capacitation and Acrosome Reaction.Front Cell Dev Biol. 2019 Nov 20;7:295. doi: 10.3389/fcell.2019.00295. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31824947 Free PMC article.
-
Membrane Potential Assessment by Fluorimetry as a Predictor Tool of Human Sperm Fertilizing Capacity.Front Cell Dev Biol. 2020 Jan 17;7:383. doi: 10.3389/fcell.2019.00383. eCollection 2019. Front Cell Dev Biol. 2020. PMID: 32010695 Free PMC article.
-
Acrosome reaction and Ca²⁺ imaging in single human spermatozoa: new regulatory roles of [Ca²⁺]i.Biol Reprod. 2014 Sep;91(3):67. doi: 10.1095/biolreprod.114.119768. Epub 2014 Aug 6. Biol Reprod. 2014. PMID: 25100708 Free PMC article.
References
-
- Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2:366. - PubMed
-
- Austin CR. The capacitation of the mammalian sperm. Nature. 1952;170:326. - PubMed
-
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–8. - PubMed
-
- Chang MC. Fertilization of rabbit ova in vitro. Nature. 1959;184 Suppl 7:466–7. - PubMed
-
- Suarez SS. Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol. 2008;52:455–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

