Myocyte proliferation in the developing heart
- PMID: 21538685
- PMCID: PMC3271704
- DOI: 10.1002/dvdy.22650
Myocyte proliferation in the developing heart
Abstract
Regulation of organ growth is critical during embryogenesis. At the cellular level, mechanisms controlling the size of individual embryonic organs include cell proliferation, differentiation, migration, and attrition through cell death. All these mechanisms play a role in cardiac morphogenesis, but experimental studies have shown that the major determinant of cardiac size during prenatal development is myocyte proliferation. As this proliferative capacity becomes severely restricted after birth, the number of cell divisions that occur during embryogenesis limits the growth potential of the postnatal heart. We summarize here current knowledge concerning regional control of myocyte proliferation as related to cardiac morphogenesis and dysmorphogenesis. There are significant spatial and temporal differences in rates of cell division, peaking during the preseptation period and then gradually decreasing toward birth. Analysis of regional rates of proliferation helps to explain the mechanics of ventricular septation, chamber morphogenesis, and the development of the cardiac conduction system. Proliferation rates are influenced by hemodynamic loading, and transduced by autocrine and paracrine signaling by means of growth factors. Understanding the biological response of the developing heart to such factors and physical forces will further our progress in engineering artificial myocardial tissues for heart repair and designing optimal treatment strategies for congenital heart disease.
Copyright © 2011 Wiley-Liss, Inc.
Figures
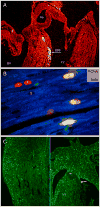
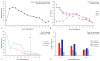
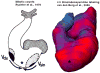
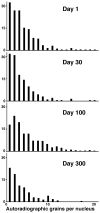
Similar articles
-
Wnt/β-catenin signaling directs the regional expansion of first and second heart field-derived ventricular cardiomyocytes.Development. 2013 Oct;140(20):4165-76. doi: 10.1242/dev.099325. Epub 2013 Sep 11. Development. 2013. PMID: 24026118 Free PMC article.
-
Numb family proteins are essential for cardiac morphogenesis and progenitor differentiation.Development. 2014 Jan;141(2):281-95. doi: 10.1242/dev.093690. Epub 2013 Dec 11. Development. 2014. PMID: 24335256 Free PMC article.
-
Foxp1 regulates cardiac outflow tract, endocardial cushion morphogenesis and myocyte proliferation and maturation.Development. 2004 Sep;131(18):4477-87. doi: 10.1242/dev.01287. Development. 2004. PMID: 15342473
-
Regulatory mechanisms of cardiac development and repair.Cardiovasc Hematol Disord Drug Targets. 2006 Jun;6(2):101-12. doi: 10.2174/187152906777441849. Cardiovasc Hematol Disord Drug Targets. 2006. PMID: 16787195 Review.
-
Uncovering the molecular and cellular mechanisms of heart development using the zebrafish.Annu Rev Genet. 2012;46:397-418. doi: 10.1146/annurev-genet-110711-155646. Epub 2012 Sep 4. Annu Rev Genet. 2012. PMID: 22974299 Free PMC article. Review.
Cited by
-
Translation of Tudor-SN, a novel terminal oligo-pyrimidine (TOP) mRNA, is regulated by the mTORC1 pathway in cardiomyocytes.RNA Biol. 2021 Jun;18(6):900-913. doi: 10.1080/15476286.2020.1827783. Epub 2020 Oct 15. RNA Biol. 2021. PMID: 33054526 Free PMC article.
-
Targeted inactivation of Cerberus like-2 leads to left ventricular cardiac hyperplasia and systolic dysfunction in the mouse.PLoS One. 2014 Jul 17;9(7):e102716. doi: 10.1371/journal.pone.0102716. eCollection 2014. PLoS One. 2014. PMID: 25033293 Free PMC article.
-
Embryonic Hyperglycemia Disrupts Myocardial Growth, Morphological Development, and Cellular Organization: An In Vivo Experimental Study.Life (Basel). 2023 Mar 13;13(3):768. doi: 10.3390/life13030768. Life (Basel). 2023. PMID: 36983924 Free PMC article.
-
Validating the Paradigm That Biomechanical Forces Regulate Embryonic Cardiovascular Morphogenesis and Are Fundamental in the Etiology of Congenital Heart Disease.J Cardiovasc Dev Dis. 2020 Jun 12;7(2):23. doi: 10.3390/jcdd7020023. J Cardiovasc Dev Dis. 2020. PMID: 32545681 Free PMC article. Review.
-
Harnessing developmental cues for cardiomyocyte production.Development. 2023 Aug 1;150(15):dev201483. doi: 10.1242/dev.201483. Epub 2023 Aug 10. Development. 2023. PMID: 37560977 Free PMC article. Review.
References
-
- Aanhaanen WT, Brons JF, Dominguez JN, Rana MS, Norden J, Airik R, Wakker V, de Gier-de Vries C, Brown NA, Kispert A, Moorman AF, Christoffels VM. The Tbx2+ primary myocardium of the atrioventricular canal forms the atrioventricular node and the base of the left ventricle. Circ Res. 2009;104:1267–1274. - PubMed
-
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. - PubMed
-
- Anversa P, Leri A, Beltrami CA, Guerra S, Kajstura J. Myocyte death and growth in the failing heart. Lab Invest. 1998;78:767–786. - PubMed
-
- Asai R, Kurihara Y, Fujisawa K, Sato T, Kawamura Y, Kokubo H, Tonami K, Nishiyama K, Uchijima Y, Miyagawa-Tomita S, Kurihara H. Endothelin receptor type A expression defines a distinct cardiac subdomain within the heart field and is later implicated in chamber myocardium formation. Development. 2010;137:3823–3833. - PubMed
-
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

