Nuclear actin polymerization is required for transcriptional reprogramming of Oct4 by oocytes
- PMID: 21536734
- PMCID: PMC3084028
- DOI: 10.1101/gad.615211
Nuclear actin polymerization is required for transcriptional reprogramming of Oct4 by oocytes
Abstract
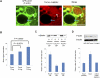
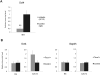
Amphibian oocytes can rapidly and efficiently reprogram the transcription of transplanted somatic nuclei. To explore the factors and mechanisms involved, we focused on nuclear actin, an especially abundant component of the oocyte's nucleus (the germinal vesicle). The existence and significance of nuclear actin has long been debated. Here, we found that nuclear actin polymerization plays an essential part in the transcriptional reactivation of the pluripotency gene Oct4 (also known as Pou5f1). We also found that an actin signaling protein, Toca-1, enhances Oct4 reactivation by regulating nuclear actin polymerization. Toca-1 overexpression has an effect on the chromatin state of transplanted nuclei, including the enhanced binding of nuclear actin to gene regulatory regions. This is the first report showing that naturally stored actin in an oocyte nucleus helps transcriptional reprogramming in a polymerization-dependent manner.
Figures

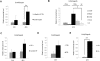


Similar articles
-
Nuclear actin in transcriptional reprogramming by oocytes: are actin nucleators key players?Cell Cycle. 2011 Sep 15;10(18):3040-1. doi: 10.4161/cc.10.18.16946. Epub 2011 Sep 15. Cell Cycle. 2011. PMID: 21869611 Free PMC article. No abstract available.
-
Characterization of somatic cell nuclear reprogramming by oocytes in which a linker histone is required for pluripotency gene reactivation.Proc Natl Acad Sci U S A. 2010 Mar 23;107(12):5483-8. doi: 10.1073/pnas.1000599107. Epub 2010 Mar 8. Proc Natl Acad Sci U S A. 2010. PMID: 20212135 Free PMC article.
-
Nuclear actin and transcriptional activation.Commun Integr Biol. 2011 Sep;4(5):582-3. doi: 10.4161/cib.4.5.16491. Epub 2011 Sep 1. Commun Integr Biol. 2011. PMID: 22046469 Free PMC article.
-
Transcriptional regulation and nuclear reprogramming: roles of nuclear actin and actin-binding proteins.Cell Mol Life Sci. 2013 Sep;70(18):3289-302. doi: 10.1007/s00018-012-1235-7. Epub 2012 Dec 29. Cell Mol Life Sci. 2013. PMID: 23275942 Free PMC article. Review.
-
Reprogramming of somatic cells and nuclei by Xenopus oocyte and egg extracts.Int J Dev Biol. 2016;60(7-8-9):289-296. doi: 10.1387/ijdb.160163at. Int J Dev Biol. 2016. PMID: 27251073 Review.
Cited by
-
Filamentous Actin in the Nucleus in Triple-Negative Breast Cancer Stem Cells: A Key to Drug-Induced Nucleolar Stress and Stemness Inhibition?J Cancer. 2024 Sep 3;15(17):5636-5642. doi: 10.7150/jca.98113. eCollection 2024. J Cancer. 2024. PMID: 39308680 Free PMC article.
-
A Role for Nuclear Actin in HDAC 1 and 2 Regulation.Sci Rep. 2016 Jun 27;6:28460. doi: 10.1038/srep28460. Sci Rep. 2016. PMID: 27345839 Free PMC article.
-
Role of dynamic nuclear deformation on genomic architecture reorganization.PLoS Comput Biol. 2019 Sep 11;15(9):e1007289. doi: 10.1371/journal.pcbi.1007289. eCollection 2019 Sep. PLoS Comput Biol. 2019. PMID: 31509522 Free PMC article.
-
Anillin regulates breast cancer cell migration, growth, and metastasis by non-canonical mechanisms involving control of cell stemness and differentiation.Breast Cancer Res. 2020 Jan 7;22(1):3. doi: 10.1186/s13058-019-1241-x. Breast Cancer Res. 2020. PMID: 31910867 Free PMC article.
-
Comparative RNAi screening identifies a conserved core metazoan actinome by phenotype.J Cell Biol. 2011 Sep 5;194(5):789-805. doi: 10.1083/jcb.201103168. J Cell Biol. 2011. PMID: 21893601 Free PMC article.
References
-
- Ambrosino C, Tarallo R, Bamundo A, Cuomo D, Franci G, Nassa G, Paris O, Ravo M, Giovane A, Zambrano N, et al. 2010. Identification of a hormone-regulated dynamic nuclear actin network associated with estrogen receptor α in human breast cancer cell nuclei. Mol Cell Proteomics 9: 1352–1367 - PMC - PubMed
-
- Astrand C, Belikov S, Wrange O 2009. Histone acetylation characterizes chromatin presetting by NF1 and Oct1 and enhances glucocorticoid receptor binding to the MMTV promoter. Exp Cell Res 315: 2604–2615 - PubMed
-
- Bettinger BT, Gilbert DM, Amberg DC 2004. Actin up in the nucleus. Nat Rev Mol Cell Biol 5: 410–415 - PubMed
-
- Bohnsack MT, Stuven T, Kuhn C, Cordes VC, Gorlich D 2006. A selective block of nuclear actin export stabilizes the giant nuclei of Xenopus oocytes. Nat Cell Biol 8: 257–263 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
