Targeted gene expression in the transgenic Aedes aegypti using the binary Gal4-UAS system
- PMID: 21536128
- PMCID: PMC3124619
- DOI: 10.1016/j.ibmb.2011.04.004
Targeted gene expression in the transgenic Aedes aegypti using the binary Gal4-UAS system
Abstract
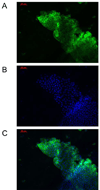
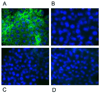
In this study, we report the establishment of the binary Gal4/UAS system for the yellow fever mosquito Aedes aegypti. We utilized the 1.8-kb 5' upstream region of the vitellogenin gene (Vg) to genetically engineer mosquito lines with the Vg-Gal4 activator and established UAS-EGFP responder transgenic mosquito lines to evaluate the binary Gal4/UAS system. The results show that the Vg-Gal4 driver leads to a high level of tissue-, stage- and sex-specific expression of the EGFP reporter in the fat body of Vg-Gal4/UAS-EGFP hybrids after blood-meal activation. In addition, the applicability of this system to study hormonal regulation of gene expression was demonstrated in in vitro organ culture experiments in which the EGFP reporter was highly activated in isolated fat bodies of previtellogenic Vg-Gal4/UAS-EGFP females incubated in the presence of 20-hydroxyecdysone (20E). Hence, this study has opened the door for further refinement of genetic tools in mosquitoes.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Regulation of the gut-specific carboxypeptidase: a study using the binary Gal4/UAS system in the mosquito Aedes aegypti.Insect Biochem Mol Biol. 2014 Nov;54:1-10. doi: 10.1016/j.ibmb.2014.08.001. Epub 2014 Aug 21. Insect Biochem Mol Biol. 2014. PMID: 25152428 Free PMC article.
-
Determination of juvenile hormone titers by means of LC-MS/MS/MS and a juvenile hormone-responsive Gal4/UAS system in Aedes aegypti mosquitoes.Insect Biochem Mol Biol. 2016 Oct;77:69-77. doi: 10.1016/j.ibmb.2016.08.003. Epub 2016 Aug 12. Insect Biochem Mol Biol. 2016. PMID: 27530057 Free PMC article.
-
Indirect control of yolk protein genes by 20-hydroxyecdysone in the fat body of the mosquito, Aedes aegypti.Insect Biochem Mol Biol. 1995 Apr;25(4):449-54. doi: 10.1016/0965-1748(94)00082-a. Insect Biochem Mol Biol. 1995. PMID: 7742832
-
Molecular biology of mosquito vitellogenesis: from basic studies to genetic engineering of antipathogen immunity.Insect Biochem Mol Biol. 2002 Oct;32(10):1275-86. doi: 10.1016/s0965-1748(02)00090-5. Insect Biochem Mol Biol. 2002. PMID: 12225918 Review.
-
Functional characterization of the cis-regulatory region for the vitellogenin gene in Plutella xylostella.Insect Mol Biol. 2020 Apr;29(2):137-147. doi: 10.1111/imb.12632. Epub 2020 Jan 8. Insect Mol Biol. 2020. PMID: 31850544 Review.
Cited by
-
Distinct roles of isoforms of the heme-liganded nuclear receptor E75, an insect ortholog of the vertebrate Rev-erb, in mosquito reproduction.Mol Cell Endocrinol. 2012 Feb 26;349(2):262-71. doi: 10.1016/j.mce.2011.11.006. Epub 2011 Nov 17. Mol Cell Endocrinol. 2012. PMID: 22115961 Free PMC article.
-
Juvenile Hormone Biosynthesis in Insects: What Is New, What Do We Know, and What Questions Remain?Int Sch Res Notices. 2014 Oct 19;2014:967361. doi: 10.1155/2014/967361. eCollection 2014. Int Sch Res Notices. 2014. PMID: 27382622 Free PMC article. Review.
-
Transposon and Transgene Tribulations in Mosquitoes: A Perspective of piRNA Proportions.DNA (Basel). 2024 Jun;4(2):104-128. doi: 10.3390/dna4020006. Epub 2024 Mar 30. DNA (Basel). 2024. PMID: 39076684 Free PMC article.
-
Organization of olfactory centres in the malaria mosquito Anopheles gambiae.Nat Commun. 2016 Oct 3;7:13010. doi: 10.1038/ncomms13010. Nat Commun. 2016. PMID: 27694947 Free PMC article.
-
Acoustic communication in insect disease vectors.Mem Inst Oswaldo Cruz. 2013;108 Suppl 1(Suppl 1):26-33. doi: 10.1590/0074-0276130390. Mem Inst Oswaldo Cruz. 2013. PMID: 24473800 Free PMC article. Review.
References
-
- Arensburger P, Megy K, Waterhouse RM, Abrudan J, Amedeo P, Antelo B, Bartholomay L, Bidwell S, Caler E, Camara F, Campbell CL, Campbell KS, Casola C, Castro MT, Chandramouliswaran I, Chapman SB, Christley S, Costas J, Eisenstadt E, Feschotte C, Fraser-Liggett C, Guigo R, Haas B, Hammond M, Hansson BS, Hemingway J, Hill SR, Howarth C, Ignell R, Kennedy RC, Kodira CD, Lobo NF, Mao C, Mayhew G, Michel K, Mori A, Liu N, Naveira H, Nene V, Nguyen N, Pearson MD, Pritham EJ, Puiu D, Qi Y, Ranson H, Ribeiro JM, Roberston HM, Severson DW, Shumway M, Stanke M, Strausberg RL, Sun C, Sutton G, Tu ZJ, Tubio JM, Unger MF, Vanlandingham DL, Vilella AJ, White O, White JR, Wondji CS, Wortman J, Zdobnov EM, Birren B, Christensen BM, Collins FH, Cornel A, Dimopoulos G, Hannick LI, Higgs S, Lanzaro GC, Lawson D, Lee NH, Muskavitch MA, Raikhel AS, Atkinson PW. Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science. 2010;330:86–88. - PMC - PubMed
-
- Attardo GM, Hansen IA, Raikhel AS. Nutritional regulation of vitellogenesis in mosquitoes: Implications for anautogeny. Insect Biochem. Mol. Biol. 2005;35:661–675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

