Latency antigen α-crystallin based vaccination imparts a robust protection against TB by modulating the dynamics of pulmonary cytokines
- PMID: 21533158
- PMCID: PMC3078913
- DOI: 10.1371/journal.pone.0018773
Latency antigen α-crystallin based vaccination imparts a robust protection against TB by modulating the dynamics of pulmonary cytokines
Abstract
Background: Efficient control of tuberculosis (TB) requires development of strategies that can enhance efficacy of the existing vaccine Mycobacterium bovis Bacille Calmette Guerin (BCG). To date only a few studies have explored the potential of latency-associated antigens to augment the immunogenicity of BCG.
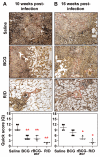
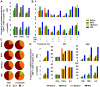
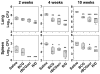
Methods/principal findings: We evaluated the protective efficacy of a heterologous prime boost approach based on recombinant BCG and DNA vaccines targeting α-crystallin, a prominent latency antigen. We show that "rBCG prime-DNA boost" strategy (R/D) confers a markedly superior protection along with reduced pathology in comparison to BCG vaccination in guinea pigs (565 fold and 45 fold reduced CFU in lungs and spleen, respectively, in comparison to BCG vaccination). In addition, R/D regimen also confers enhanced protection in mice. Our results in guinea pig model show a distinct association of enhanced protection with an increased level of interleukin (IL)12 and a simultaneous increase in immuno-regulatory cytokines such as transforming growth factor (TGF)β and IL10 in lungs. The T cell effector functions, which could not be measured in guinea pigs due to technical limitations, were characterized in mice by multi-parameter flow cytometry. We show that R/D regimen elicits a heightened multi-functional CD4 Th1 cell response leading to enhanced protection.
Conclusions/significance: These results clearly indicate the superiority of α-crystallin based R/D regimen over BCG. Our observations from guinea pig studies indicate a crucial role of IL12, IL10 and TGFβ in vaccine-induced protection. Further, characterization of T cell responses in mice demonstrates that protection against TB is predictable by the frequency of CD4 T cells simultaneously producing interferon (IFN)γ, tumor necrosis factor (TNF)α and IL2. We anticipate that this study will not only contribute toward the development of a superior alternative to BCG, but will also stimulate designing of TB vaccines based on latency antigens.
Conflict of interest statement
Figures
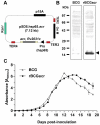
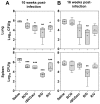
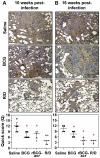

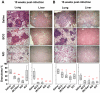

Similar articles
-
A booster vaccine expressing a latency-associated antigen augments BCG induced immunity and confers enhanced protection against tuberculosis.PLoS One. 2011;6(8):e23360. doi: 10.1371/journal.pone.0023360. Epub 2011 Aug 17. PLoS One. 2011. PMID: 21858087 Free PMC article.
-
Heterologous Boost Following Mycobacterium bovis BCG Reduces the Late Persistent, Rather Than the Early Stage of Intranasal Tuberculosis Challenge Infection.Front Immunol. 2018 Oct 30;9:2439. doi: 10.3389/fimmu.2018.02439. eCollection 2018. Front Immunol. 2018. PMID: 30425711 Free PMC article.
-
Enhanced and enduring protection against tuberculosis by recombinant BCG-Ag85C and its association with modulation of cytokine profile in lung.PLoS One. 2008;3(12):e3869. doi: 10.1371/journal.pone.0003869. Epub 2008 Dec 4. PLoS One. 2008. PMID: 19052643 Free PMC article.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Is interferon-gamma the right marker for bacille Calmette-Guérin-induced immune protection? The missing link in our understanding of tuberculosis immunology.Clin Exp Immunol. 2012 Sep;169(3):213-9. doi: 10.1111/j.1365-2249.2012.04614.x. Clin Exp Immunol. 2012. PMID: 22861360 Free PMC article. Review.
Cited by
-
A new DNA vaccine expressing HspX-PPE44-EsxV fusion antigens of Mycobacterium tuberculosis induced strong immune responses.Iran J Basic Med Sci. 2020 Jul;23(7):909-914. doi: 10.22038/ijbms.2020.38521.9171. Iran J Basic Med Sci. 2020. PMID: 32774813 Free PMC article.
-
Pro- and anti-inflammatory cytokines against Rv2031 are elevated during latent tuberculosis: a study in cohorts of tuberculosis patients, household contacts and community controls in an endemic setting.PLoS One. 2015 Apr 21;10(4):e0124134. doi: 10.1371/journal.pone.0124134. eCollection 2015. PLoS One. 2015. PMID: 25897840 Free PMC article.
-
A booster vaccine expressing a latency-associated antigen augments BCG induced immunity and confers enhanced protection against tuberculosis.PLoS One. 2011;6(8):e23360. doi: 10.1371/journal.pone.0023360. Epub 2011 Aug 17. PLoS One. 2011. PMID: 21858087 Free PMC article.
-
Prime-boost approaches to tuberculosis vaccine development.Expert Rev Vaccines. 2012 Oct;11(10):1221-33. doi: 10.1586/erv.12.94. Expert Rev Vaccines. 2012. PMID: 23176655 Free PMC article. Review.
-
Evaluating the Performance of PPE44, HSPX, ESAT-6 and CFP-10 Factors in Tuberculosis Subunit Vaccines.Curr Microbiol. 2022 Jul 19;79(9):260. doi: 10.1007/s00284-022-02949-8. Curr Microbiol. 2022. PMID: 35852636 Free PMC article. Review.
References
-
- Smith PGaMAR., editor. Epidemiology of tuberculosis. Washington, D.C: American Society for Microbiology Press; 1994. 637
-
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

