Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells
- PMID: 21532990
- PMCID: PMC3080917
- DOI: 10.1371/journal.pone.0019183
Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells
Abstract
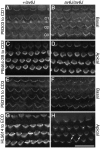
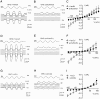
Immunocytochemical studies have shown that protocadherin-15 (PCDH15) and cadherin-23 (CDH23) are associated with tip links, structures thought to gate the mechanotransducer channels of hair cells in the sensory epithelia of the inner ear. The present report describes functional and structural analyses of hair cells from Pcdh15(av3J) (av3J), Pcdh15(av6J) (av6J) and Cdh23(v2J) (v2J) mice. The av3J and v2J mice carry point mutations that are predicted to introduce premature stop codons in the transcripts for Pcdh15 and Cdh23, respectively, and av6J mice have an in-frame deletion predicted to remove most of the 9th cadherin ectodomain from PCDH15. Severe disruption of hair-bundle morphology is observed throughout the early-postnatal cochlea in av3J/av3J and v2J/v2J mice. In contrast, only mild-to-moderate bundle disruption is evident in the av6J/av6J mice. Hair cells from av3J/av3J mice are unaffected by aminoglycosides and fail to load with [(3)H]-gentamicin or FM1-43, compounds that permeate the hair cell's mechanotransducer channels. In contrast, hair cells from av6J/av6J mice load with both FM1-43 and [(3)H]-gentamicin, and are aminoglycoside sensitive. Transducer currents can be recorded from hair cells of all three mutants but are reduced in amplitude in all mutants and have abnormal directional sensitivity in the av3J/av3J and v2J/v2J mutants. Scanning electron microscopy of early postnatal cochlear hair cells reveals tip-link like links in av6J/av6J mice, substantially reduced numbers of links in the av3J/av3J mice and virtually none in the v2J/v2J mice. Analysis of mature vestibular hair bundles reveals an absence of tip links in the av3J/av3J and v2J/v2J mice and a reduction in av6J/av6J mice. These results therefore provide genetic evidence consistent with PCDH15 and CDH23 being part of the tip-link complex and necessary for normal mechanotransduction.
Conflict of interest statement
Figures









Similar articles
-
Noddy, a mouse harboring a missense mutation in protocadherin-15, reveals the impact of disrupting a critical interaction site between tip-link cadherins in inner ear hair cells.J Neurosci. 2013 Mar 6;33(10):4395-404. doi: 10.1523/JNEUROSCI.4514-12.2013. J Neurosci. 2013. PMID: 23467356 Free PMC article.
-
Development and regeneration of sensory transduction in auditory hair cells requires functional interaction between cadherin-23 and protocadherin-15.J Neurosci. 2010 Aug 25;30(34):11259-69. doi: 10.1523/JNEUROSCI.1949-10.2010. J Neurosci. 2010. PMID: 20739546 Free PMC article.
-
Tuning Inner-Ear Tip-Link Affinity Through Alternatively Spliced Variants of Protocadherin-15.Biochemistry. 2018 Mar 20;57(11):1702-1710. doi: 10.1021/acs.biochem.7b01075. Epub 2018 Mar 6. Biochemistry. 2018. PMID: 29443515 Free PMC article.
-
The tip-link molecular complex of the auditory mechano-electrical transduction machinery.Hear Res. 2015 Dec;330(Pt A):10-7. doi: 10.1016/j.heares.2015.05.005. Epub 2015 Jun 3. Hear Res. 2015. PMID: 26049141 Review.
-
Cadherins and mechanotransduction by hair cells.Curr Opin Cell Biol. 2008 Oct;20(5):557-66. doi: 10.1016/j.ceb.2008.06.004. Epub 2008 Jul 30. Curr Opin Cell Biol. 2008. PMID: 18619539 Free PMC article. Review.
Cited by
-
Noddy, a mouse harboring a missense mutation in protocadherin-15, reveals the impact of disrupting a critical interaction site between tip-link cadherins in inner ear hair cells.J Neurosci. 2013 Mar 6;33(10):4395-404. doi: 10.1523/JNEUROSCI.4514-12.2013. J Neurosci. 2013. PMID: 23467356 Free PMC article.
-
Is TMC1 the Hair Cell Mechanotransducer Channel?Biophys J. 2016 Jul 12;111(1):3-9. doi: 10.1016/j.bpj.2016.05.032. Biophys J. 2016. PMID: 27410728 Free PMC article. Review.
-
CLIC5 stabilizes membrane-actin filament linkages at the base of hair cell stereocilia in a molecular complex with radixin, taperin, and myosin VI.Cytoskeleton (Hoboken). 2014 Jan;71(1):61-78. doi: 10.1002/cm.21159. Epub 2013 Dec 10. Cytoskeleton (Hoboken). 2014. PMID: 24285636 Free PMC article.
-
Mechanically Gated Ion Channels in Mammalian Hair Cells.Front Cell Neurosci. 2018 Apr 11;12:100. doi: 10.3389/fncel.2018.00100. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29755320 Free PMC article. Review.
-
Control of stereocilia length during development of hair bundles.PLoS Biol. 2023 Apr 3;21(4):e3001964. doi: 10.1371/journal.pbio.3001964. eCollection 2023 Apr. PLoS Biol. 2023. PMID: 37011103 Free PMC article.
References
-
- Leibovici M, Safieddine S, Petit C. Mouse models for human hereditary deafness. Curr Top Dev Biol. 2008;84:385–429. - PubMed
-
- Dror AA, Avraham KB. Hearing loss: mechanisms revealed by genetics and cell biology. Annu Rev Genet. 2009;43:411–437. - PubMed
-
- Friedman LM, Dror AA, Avraham KB. Mouse models to study inner ear development and hereditary hearing loss. Int J Dev Biol. 2007;51:609–631. - PubMed
-
- Pickles JO, Comis SD, Osborne MP. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res. 1984;15:103–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

