Cdc42 in oncogenic transformation, invasion, and tumorigenesis
- PMID: 21515363
- PMCID: PMC3115433
- DOI: 10.1016/j.cellsig.2011.04.001
Cdc42 in oncogenic transformation, invasion, and tumorigenesis
Abstract
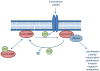
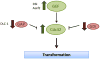
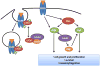
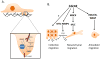
The Rho family of GTPases represents a class of Ras-related signaling molecules often deregulated in cancer. Rho GTPases switch from a GDP-bound, inactive state to a GTP-bound, active state in response to extracellular stimuli such as mitogens and extracellular matrix. In addition, Rho GTPase signaling can be altered in response to cell intrinsic factors such as changes in oncogenic or tumor suppressor signaling. In their active form, these proteins bind to a number of effector molecules, activating signaling cascades which regulate a variety of cellular processes including cytoskeletal reorganization, cell cycle progression, cell polarity and transcription. Here, we focus on one Rho family member, Cdc42, which is overexpressed in a number of human cancers. Consistent with a role in the promotion of tumorigenesis, activating mutations in Cdc42 and guanine nucleotide exchange factors are transforming, while inhibition of Cdc42 activity can impinge on cellular transformation following the activation of oncoproteins or loss of tumor suppressor function. Furthermore, Cdc42 activity has been implicated in the invasive phenotype which characterizes tumor metastasis, further suggesting that Cdc42 may be a useful target for therapeutic intervention. However, several recent studies in mice have unveiled a putative tumor suppressor function of Cdc42 in several tissue types which may involve cell polarity maintenance, suggesting that the role of Cdc42 in cancer development is complex and may be cell type specific.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
-
Targeting Cdc42 in cancer.Expert Opin Ther Targets. 2013 Nov;17(11):1263-73. doi: 10.1517/14728222.2013.828037. Epub 2013 Aug 19. Expert Opin Ther Targets. 2013. PMID: 23957315 Free PMC article. Review.
-
Influencing cellular transformation by modulating the rates of GTP hydrolysis by Cdc42.Biochemistry. 2006 Jun 27;45(25):7750-62. doi: 10.1021/bi060365h. Biochemistry. 2006. PMID: 16784226
-
BRAF and RAS oncogenes regulate Rho GTPase pathways to mediate migration and invasion properties in human colon cancer cells: a comparative study.Mol Cancer. 2011 Sep 23;10:118. doi: 10.1186/1476-4598-10-118. Mol Cancer. 2011. PMID: 21943101 Free PMC article.
-
Cellular signaling for activation of Rho GTPase Cdc42.Cell Signal. 2008 Nov;20(11):1927-34. doi: 10.1016/j.cellsig.2008.05.002. Epub 2008 May 16. Cell Signal. 2008. PMID: 18558478 Review.
Cited by
-
WNT-5A triggers Cdc42 activation leading to an ERK1/2 dependent decrease in MMP9 activity and invasive migration of breast cancer cells.Mol Oncol. 2013 Oct;7(5):870-83. doi: 10.1016/j.molonc.2013.04.005. Epub 2013 Apr 28. Mol Oncol. 2013. PMID: 23727359 Free PMC article.
-
The Landscape of Signaling Pathways and Proteasome Inhibitors Combinations in Multiple Myeloma.Cancers (Basel). 2021 Mar 11;13(6):1235. doi: 10.3390/cancers13061235. Cancers (Basel). 2021. PMID: 33799793 Free PMC article. Review.
-
Subversion of Ras Small GTPases in Cutaneous Melanoma Aggressiveness.Front Cell Dev Biol. 2020 Sep 23;8:575223. doi: 10.3389/fcell.2020.575223. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33072757 Free PMC article. Review.
-
Integrinβ1 modulates tumour resistance to gemcitabine and serves as an independent prognostic factor in pancreatic adenocarcinomas.Tumour Biol. 2016 Sep;37(9):12315-12327. doi: 10.1007/s13277-016-5061-7. Epub 2016 Jun 11. Tumour Biol. 2016. PMID: 27289231
-
Role of Circular RNA in Kidney-Related Diseases.Front Pharmacol. 2021 Mar 11;12:615882. doi: 10.3389/fphar.2021.615882. eCollection 2021. Front Pharmacol. 2021. PMID: 33776764 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

