Reversal of gastrointestinal carcinoma-induced immunosuppression and induction of antitumoural immunity by a combination of cyclophosphamide and gene transfer of IL-12
- PMID: 21515097
- PMCID: PMC5528288
- DOI: 10.1016/j.molonc.2011.03.007
Reversal of gastrointestinal carcinoma-induced immunosuppression and induction of antitumoural immunity by a combination of cyclophosphamide and gene transfer of IL-12
Abstract
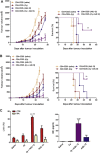
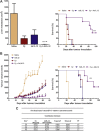
Immunotherapy-based strategies for gastrointestinal carcinomas (GIC) have been exploited so far, but these approaches have to face strong mechanisms of immune escape induced by tumours. We previously demonstrated that sub-therapeutic doses of an adenovirus expressing IL-12 genes (AdIL-12) mediated a potent antitumour effect against subcutaneous (s.c.) colorectal carcinomas (CRC) in mice pre-treated with low doses of cyclophosphamide (Cy). In our study we used this combination to assess its impact on the immunosuppressive microenvironment. In s.c. CRC model we demonstrated that non-responder mice failed to decrease Tregs in tumour, spleen and peripheral blood. Reconstitution of Tregs into tumour-bearing mice treated with combined therapy abolished the antitumoural effect. In addition, Cy + AdIL-12 modified Tregs functionality by inhibiting the in vitro secretion of IL-10 and TGF-β and their ability to inhibit dendritic cells activation. Combined treatment decreased the number of myeloid-derived suppressor cells (MDSCs) in comparison to non-treated mice and, interestingly, administration of Tregs restored splenic MDSCs population. Furthermore, combined therapy potently generated specific cytotoxic IFN-γ-secreting CD4+ T cells able to eradicate established CRC tumours after adoptive transfer. Finally, we evaluated the combination on disseminated CRC and pancreatic carcinoma (PC). Cy + AdIL-12 were able to eradicate liver metastatic CRC (47%) and PC tumour nodules (40%) and to prolong animal survival. The results of this study support the hypothesis that Cy + AdIL-12 might be a valid immunotherapeutic strategy for advanced GIC.
Copyright © 2011 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
A novel synergistic combination of cyclophosphamide and gene transfer of interleukin-12 eradicates colorectal carcinoma in mice.Clin Cancer Res. 2009 Dec 1;15(23):7256-65. doi: 10.1158/1078-0432.CCR-09-1861. Epub 2009 Nov 17. Clin Cancer Res. 2009. PMID: 19920110
-
Temporary elimination of IL-10 enhanced the effectiveness of cyclophosphamide and BMDC-based therapy by decrease of the suppressor activity of MDSCs and activation of antitumour immune response.Immunobiology. 2015 Mar;220(3):389-98. doi: 10.1016/j.imbio.2014.10.009. Epub 2014 Oct 23. Immunobiology. 2015. PMID: 25454807
-
Tumor Microenvironment Remodeling by 4-Methylumbelliferone Boosts the Antitumor Effect of Combined Immunotherapy in Murine Colorectal Carcinoma.Mol Ther. 2015 Sep;23(9):1444-55. doi: 10.1038/mt.2015.112. Epub 2015 Jun 24. Mol Ther. 2015. PMID: 26105158 Free PMC article.
-
Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective.Cancer Res. 2012 Jul 15;72(14):3439-44. doi: 10.1158/0008-5472.CAN-11-3912. Epub 2012 Jul 3. Cancer Res. 2012. PMID: 22761338 Free PMC article. Review.
-
Combined therapy for gastrointestinal carcinomas: exploiting synergies between gene therapy and classical chemo-radiotherapy.Curr Gene Ther. 2015;15(2):151-60. doi: 10.2174/1566523214666141224095757. Curr Gene Ther. 2015. PMID: 25537776 Review.
Cited by
-
Relevance of Regulatory T Cells during Colorectal Cancer Development.Cancers (Basel). 2020 Jul 14;12(7):1888. doi: 10.3390/cancers12071888. Cancers (Basel). 2020. PMID: 32674255 Free PMC article. Review.
-
4Mu Decreases CD47 Expression on Hepatic Cancer Stem Cells and Primes a Potent Antitumor T Cell Response Induced by Interleukin-12.Mol Ther. 2018 Dec 5;26(12):2738-2750. doi: 10.1016/j.ymthe.2018.09.012. Epub 2018 Sep 18. Mol Ther. 2018. PMID: 30301668 Free PMC article.
-
Multivalent presentation of MPL by porous silicon microparticles favors T helper 1 polarization enhancing the anti-tumor efficacy of doxorubicin nanoliposomes.PLoS One. 2014 Apr 15;9(4):e94703. doi: 10.1371/journal.pone.0094703. eCollection 2014. PLoS One. 2014. PMID: 24736547 Free PMC article.
-
How the Tumor Micromilieu Modulates the Recruitment and Activation of Colorectal Cancer-Infiltrating Lymphocytes.Biomedicines. 2022 Nov 15;10(11):2940. doi: 10.3390/biomedicines10112940. Biomedicines. 2022. PMID: 36428508 Free PMC article. Review.
-
Rapamycin enhances adenovirus-mediated cancer imaging and therapy in pre-immunized murine hosts.PLoS One. 2013 Sep 2;8(9):e73650. doi: 10.1371/journal.pone.0073650. eCollection 2013. PLoS One. 2013. PMID: 24023896 Free PMC article.
References
-
- Alaniz, L. , Rizzo, M. , Malvicini, M. , Jaunarena, J. , Avella, D. , Atorrasagasti, C. , Aquino, J.B. , Garcia, M. , Matar, P. , Silva, M. , Mazzolini, G. , 2009. Low molecular weight hyaluronan inhibits colorectal carcinoma growth by decreasing tumor cell proliferation and stimulating immune response. Cancer Lett.. 278, 9–16. - PubMed
-
- Almand, B. , Clark, J.I. , Nikitina, E. , van Beynen, J. , English, N.R. , Knight, S.C. , Carbone, D.P. , Gabrilovich, D.I. , 2001. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol.. 166, 678–689. - PubMed
-
- Appay, V. , Zaunders, J.J. , Papagno, L. , Sutton, J. , Jaramillo, A. , Waters, A. , Easterbrook, P. , Grey, P. , Smith, D. , McMichael, A.J. , Cooper, D.A. , Rowland-Jones, S.L. , Kelleher, A.D. , 2002. Characterization of CD4(+) CTLs ex vivo. J. Immunol.. 168, 5954–5958. - PubMed
-
- Barajas, M. , Mazzolini, G. , Genove, G. , Bilbao, R. , Narvaiza, I. , Schmitz, V. , Sangro, B. , Melero, I. , Qian, C. , Prieto, J. , 2001. Gene therapy of orthotopic hepatocellular carcinoma in rats using adenovirus coding for interleukin 12. Hepatology. 33, 52–61. - PubMed
-
- Bell, D. , Chomarat, P. , Broyles, D. , Netto, G. , Harb, G.M. , Lebecque, S. , Valladeau, J. , Davoust, J. , Palucka, K.A. , Banchereau, J. , 1999. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J. Exp. Med.. 190, 1417–1426. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

