Amphiregulin exosomes increase cancer cell invasion
- PMID: 21514161
- PMCID: PMC3417320
- DOI: 10.1016/j.cub.2011.03.043
Amphiregulin exosomes increase cancer cell invasion
Abstract
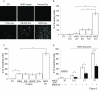
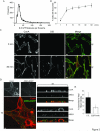
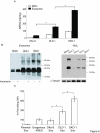
Autocrine, paracrine, and juxtacrine are recognized modes of action for mammalian EGFR ligands including EGF, TGF-α (TGFα), amphiregulin (AREG), heparin-binding EGF-like growth factor (HB-EGF), betacellulin, epiregulin, and epigen. We identify a new mode of EGFR ligand signaling via exosomes. Human breast and colorectal cancer cells release exosomes containing full-length, signaling-competent EGFR ligands. Exosomes isolated from MDCK cells expressing individual full-length EGFR ligands displayed differential activities; AREG exosomes increased invasiveness of recipient breast cancer cells 4-fold over TGFα or HB-EGF exosomes and 5-fold over equivalent amounts of recombinant AREG. Exosomal AREG displayed significantly greater membrane stability than TGFα or HB-EGF. An average of 24 AREG molecules are packaged within an individual exosome, and AREG exosomes are rapidly internalized by recipient cells. Whether the composition and behavior of exosomes differ between nontransformed and transformed cells is unknown. Exosomes from DLD-1 colon cancer cells with a mutant KRAS allele exhibited both higher AREG levels and greater invasive potential than exosomes from isogenically matched, nontransformed cells in which mutant KRAS was eliminated by homologous recombination. We speculate that EGFR ligand signaling via exosomes might contribute to diverse cancer phenomena such as field effect and priming of the metastatic niche.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
ErbB/HER ligands in human breast cancer, and relationships with their receptors, the bio-pathological features and prognosis.Ann Oncol. 2008 Jan;19(1):73-80. doi: 10.1093/annonc/mdm431. Epub 2007 Oct 24. Ann Oncol. 2008. PMID: 17962208
-
Autocrine-derived epidermal growth factor receptor ligands contribute to recruitment of tumor-associated macrophage and growth of basal breast cancer cells in vivo.Oncol Res. 2013;20(7):303-17. doi: 10.3727/096504013x13639794277761. Oncol Res. 2013. PMID: 23879171 Free PMC article.
-
Leptin stimulates the proliferation of human oesophageal adenocarcinoma cells via HB-EGF and Tgfalpha mediated transactivation of the epidermal growth factor receptor.Br J Biomed Sci. 2008;65(3):121-7. doi: 10.1080/09674845.2008.11732814. Br J Biomed Sci. 2008. PMID: 18986098
-
TACE/ADAM17 processing of EGFR ligands indicates a role as a physiological convertase.Ann N Y Acad Sci. 2003 May;995:22-38. doi: 10.1111/j.1749-6632.2003.tb03207.x. Ann N Y Acad Sci. 2003. PMID: 12814936 Review.
-
The ADAM17-amphiregulin-EGFR axis in mammary development and cancer.J Mammary Gland Biol Neoplasia. 2008 Jun;13(2):181-94. doi: 10.1007/s10911-008-9084-6. Epub 2008 May 10. J Mammary Gland Biol Neoplasia. 2008. PMID: 18470483 Free PMC article. Review.
Cited by
-
CD97 promotes gastric cancer cell proliferation and invasion through exosome-mediated MAPK signaling pathway.World J Gastroenterol. 2015 May 28;21(20):6215-28. doi: 10.3748/wjg.v21.i20.6215. World J Gastroenterol. 2015. PMID: 26034356 Free PMC article.
-
Extracellular vesicles secreted by highly metastatic clonal variants of osteosarcoma preferentially localize to the lungs and induce metastatic behaviour in poorly metastatic clones.Oncotarget. 2016 Jul 12;7(28):43570-43587. doi: 10.18632/oncotarget.9781. Oncotarget. 2016. PMID: 27259278 Free PMC article.
-
Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS.Mol Cell Proteomics. 2013 Feb;12(2):343-55. doi: 10.1074/mcp.M112.022806. Epub 2012 Nov 15. Mol Cell Proteomics. 2013. PMID: 23161513 Free PMC article.
-
Recent advances of small extracellular vesicle biomarkers in breast cancer diagnosis and prognosis.Mol Cancer. 2023 Feb 16;22(1):33. doi: 10.1186/s12943-023-01741-x. Mol Cancer. 2023. PMID: 36797736 Free PMC article. Review.
-
Dickkopf-1 and Amphiregulin as Novel Biomarkers and Potential Therapeutic Targets in Hepatocellular Carcinoma.Int J Hematol Oncol Stem Cell Res. 2019 Jul 1;13(3):153-163. Int J Hematol Oncol Stem Cell Res. 2019. PMID: 31649806 Free PMC article.
References
-
- Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal. 2005;17:1183–1193. - PubMed
-
- Chung E, Graves-Deal R, Franklin JL, Coffey RJ. Differential effects of amphiregulin and TGF-alpha on the morphology of MDCK cells. Exp Cell Res. 2005;309:149–160. - PubMed
-
- Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2–13. - PubMed
-
- Schneider MR, Wolf E. The epidermal growth factor receptor ligands at a glance. J Cell Physiol. 2009;218:460–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA46413/CA/NCI NIH HHS/United States
- P50 CA095103-10/CA/NCI NIH HHS/United States
- P30 CA68485/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R01 DK075555/DK/NIDDK NIH HHS/United States
- R25 CA092043/CA/NCI NIH HHS/United States
- P50 95103/PHS HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- 1R25 CA92043/CA/NCI NIH HHS/United States
- R01 DK075555-04/DK/NIDDK NIH HHS/United States
- P20 GM072048/GM/NIGMS NIH HHS/United States
- DK058404/DK/NIDDK NIH HHS/United States
- R01 CA046413-23/CA/NCI NIH HHS/United States
- T32 HD007502/HD/NICHD NIH HHS/United States
- T32 CA009582/CA/NCI NIH HHS/United States
- P50 CA095103/CA/NCI NIH HHS/United States
- GM72048/GM/NIGMS NIH HHS/United States
- R01 CA046413/CA/NCI NIH HHS/United States
- DK075555/DK/NIDDK NIH HHS/United States
- R01 CA163563/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

