Myeloma xenograft destruction by a nonviral vector delivering oncolytic infectious nucleic acid
- PMID: 21505425
- PMCID: PMC3129797
- DOI: 10.1038/mt.2011.68
Myeloma xenograft destruction by a nonviral vector delivering oncolytic infectious nucleic acid
Abstract
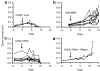
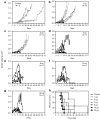
The feasibility of using a nonviral vector formulation to initiate an oncolytic viral infection has not been previously demonstrated. We therefore sought to determine whether infectious nucleic acid (INA) could be used in place of virus particles to initiate an oncolytic picornavirus infection in vivo. Infectious RNA encoding coxsackievirus A21 (CVA21) was transcribed from plasmid DNA using T7 polymerase. Within 48 hours of injecting this RNA into KAS6/1 myeloma xenografts, high titers of infectious CVA21 virions were detected in the bloodstream. Tumors regressed rapidly thereafter and mice developed signs of myositis. At euthanasia, CVA21 was recovered from regressing tumors and from skeletal muscles. Treatment outcomes were comparable following intratumoral injection of naked RNA or fully infectious CVA21 virus. Dose-response studies showed that an effective oncolytic infection could be established by intratumoral injection of 1 µg of infectious RNA. The oncolytic infection could also be initiated by intravenous injection of infectious RNA. Our study demonstrates that INA is a highly promising alternative drug formulation for oncolytic virotherapy.
Figures


Similar articles
-
Oncolytic Activity of Targeted Picornaviruses Formulated as Synthetic Infectious RNA.Mol Ther Oncolytics. 2020 May 19;17:484-495. doi: 10.1016/j.omto.2020.05.003. eCollection 2020 Jun 26. Mol Ther Oncolytics. 2020. PMID: 32529026 Free PMC article.
-
Engineering Oncolytic Coxsackievirus A21 with Small Transgenes and Enabling Cell-Mediated Virus Delivery by Integrating Viral cDNA into the Genome.J Virol. 2023 May 31;97(5):e0030923. doi: 10.1128/jvi.00309-23. Epub 2023 Apr 18. J Virol. 2023. PMID: 37070982 Free PMC article.
-
Enhanced oncolysis mediated by Coxsackievirus A21 in combination with doxorubicin hydrochloride.Invest New Drugs. 2012 Apr;30(2):568-81. doi: 10.1007/s10637-010-9614-0. Epub 2010 Dec 21. Invest New Drugs. 2012. PMID: 21170760
-
[Oncolytic coxsackievirus therapy as an immunostimulator].Rinsho Ketsueki. 2017;58(8):977-982. doi: 10.11406/rinketsu.58.977. Rinsho Ketsueki. 2017. PMID: 28883283 Review. Japanese.
-
Applications of coxsackievirus A21 in oncology.Oncolytic Virother. 2014 Apr 10;3:47-55. doi: 10.2147/OV.S56322. eCollection 2014. Oncolytic Virother. 2014. PMID: 27512662 Free PMC article. Review.
Cited by
-
miRNA-Mediated Mechanisms in the Generation of Effective and Safe Oncolytic Viruses.Pharmaceutics. 2024 Jul 25;16(8):986. doi: 10.3390/pharmaceutics16080986. Pharmaceutics. 2024. PMID: 39204331 Free PMC article. Review.
-
Viral Vectors in Gene Therapy: Where Do We Stand in 2023?Viruses. 2023 Mar 7;15(3):698. doi: 10.3390/v15030698. Viruses. 2023. PMID: 36992407 Free PMC article. Review.
-
Virus-inspired strategies for cancer therapy.Semin Cancer Biol. 2022 Nov;86(Pt 3):1143-1157. doi: 10.1016/j.semcancer.2021.06.021. Epub 2021 Jun 26. Semin Cancer Biol. 2022. PMID: 34182141 Free PMC article. Review.
-
Oncolytic Activity of Targeted Picornaviruses Formulated as Synthetic Infectious RNA.Mol Ther Oncolytics. 2020 May 19;17:484-495. doi: 10.1016/j.omto.2020.05.003. eCollection 2020 Jun 26. Mol Ther Oncolytics. 2020. PMID: 32529026 Free PMC article.
-
Developing Picornaviruses for Cancer Therapy.Cancers (Basel). 2019 May 16;11(5):685. doi: 10.3390/cancers11050685. Cancers (Basel). 2019. PMID: 31100962 Free PMC article. Review.
References
-
- Bernt KM, Ni S, Gaggar A, Li ZY, Shayakhmetov DM., and, Lieber A. The effect of sequestration by nontarget tissues on anti-tumor efficacy of systemically applied, conditionally replicating adenovirus vectors. Mol Ther. 2003;8:746–755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

