Tetherin does not significantly restrict dendritic cell-mediated HIV-1 transmission and its expression is upregulated by newly synthesized HIV-1 Nef
- PMID: 21504576
- PMCID: PMC3108291
- DOI: 10.1186/1742-4690-8-26
Tetherin does not significantly restrict dendritic cell-mediated HIV-1 transmission and its expression is upregulated by newly synthesized HIV-1 Nef
Abstract
Background: Dendritic cells (DCs) are among the first cells to encounter HIV-1 and play important roles in viral transmission and pathogenesis. Immature DCs allow productive HIV-1 replication and long-term viral dissemination. The pro-inflammatory factor lipopolysaccharide (LPS) induces DC maturation and enhances the efficiency of DC-mediated HIV-1 transmission. Type I interferon (IFN) partially inhibits HIV-1 replication and cell-cell transmission in CD4+ T cells and macrophages. Tetherin is a type I IFN-inducible restriction factor that blocks HIV-1 release and modulates CD4+ T cell-mediated cell-to-cell transmission of HIV-1. However, the role of type I IFN and tetherin in HIV-1 infection of DCs and DC-mediated viral transmission remains unknown.
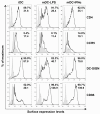
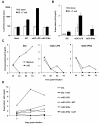
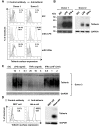
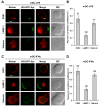
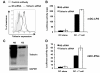
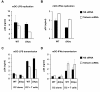
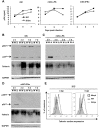
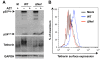
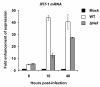
Results: We demonstrated that IFN-alpha (IFNα)-induced mature DCs restricted HIV-1 replication and trans-infection of CD4+ T cells. Tetherin expression in monocyte-derived immature DCs was undetectable or very low. High levels of tetherin were transiently expressed in LPS- and IFNα-induced mature DCs, while HIV-1 localized into distinct patches in these DCs. Knockdown of induced tetherin in LPS- or IFNα-matured DCs modestly enhanced HIV-1 transmission to CD4+ T cells, but had no significant effect on wild-type HIV-1 replication in mature DCs. Intriguingly, we found that HIV-1 replication in immature DCs induced significant tetherin expression in a Nef-dependent manner.
Conclusions: The restriction of HIV-1 replication and transmission in IFNα-induced mature DCs indicates a potent anti-HIV-1 response; however, high levels of tetherin induced in mature DCs cannot significantly restrict wild-type HIV-1 release and DC-mediated HIV-1 transmission. Nef-dependent tetherin induction in HIV-1-infected immature DCs suggests an innate immune response of DCs to HIV-1 infection.
Figures
Similar articles
-
TLR-4 engagement of dendritic cells confers a BST-2/tetherin-mediated restriction of HIV-1 infection to CD4+ T cells across the virological synapse.Retrovirology. 2013 Jan 11;10:6. doi: 10.1186/1742-4690-10-6. Retrovirology. 2013. PMID: 23311681 Free PMC article.
-
HIV-1 Nef enhances dendritic cell-mediated viral transmission to CD4+ T cells and promotes T-cell activation.PLoS One. 2012;7(3):e34521. doi: 10.1371/journal.pone.0034521. Epub 2012 Mar 30. PLoS One. 2012. PMID: 22479639 Free PMC article.
-
Endogenously expressed nef uncouples cytokine and chemokine production from membrane phenotypic maturation in dendritic cells.J Immunol. 2002 Oct 15;169(8):4172-82. doi: 10.4049/jimmunol.169.8.4172. J Immunol. 2002. PMID: 12370346
-
Emerging role of the host restriction factor tetherin in viral immune sensing.J Mol Biol. 2013 Dec 13;425(24):4956-64. doi: 10.1016/j.jmb.2013.09.029. Epub 2013 Sep 26. J Mol Biol. 2013. PMID: 24075872 Review.
-
Tetherin/BST-2: Restriction Factor or Immunomodulator?Curr HIV Res. 2016;14(3):235-46. doi: 10.2174/1570162x14999160224102752. Curr HIV Res. 2016. PMID: 26957198 Free PMC article. Review.
Cited by
-
HIV cell-to-cell transmission: effects on pathogenesis and antiretroviral therapy.Trends Microbiol. 2015 May;23(5):289-95. doi: 10.1016/j.tim.2015.02.003. Epub 2015 Mar 9. Trends Microbiol. 2015. PMID: 25766144 Free PMC article. Review.
-
Restriction of Retroviral Replication by Tetherin/BST-2.Mol Biol Int. 2012;2012:424768. doi: 10.1155/2012/424768. Epub 2012 Jul 2. Mol Biol Int. 2012. PMID: 22811908 Free PMC article.
-
TLR-4 engagement of dendritic cells confers a BST-2/tetherin-mediated restriction of HIV-1 infection to CD4+ T cells across the virological synapse.Retrovirology. 2013 Jan 11;10:6. doi: 10.1186/1742-4690-10-6. Retrovirology. 2013. PMID: 23311681 Free PMC article.
-
Development of human dendritic cells and their role in HIV infection: antiviral immunity versus HIV transmission.Front Microbiol. 2013 Jul 9;4:178. doi: 10.3389/fmicb.2013.00178. eCollection 2013. Front Microbiol. 2013. PMID: 23847602 Free PMC article.
-
Cellular and viral mechanisms of HIV-1 transmission mediated by dendritic cells.Adv Exp Med Biol. 2013;762:109-30. doi: 10.1007/978-1-4614-4433-6_4. Adv Exp Med Biol. 2013. PMID: 22975873 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

