TLR4 signaling via MyD88 and TRIF differentially shape the CD4+ T cell response to Porphyromonas gingivalis hemagglutinin B
- PMID: 21498664
- PMCID: PMC3809913
- DOI: 10.4049/jimmunol.1003192
TLR4 signaling via MyD88 and TRIF differentially shape the CD4+ T cell response to Porphyromonas gingivalis hemagglutinin B
Abstract
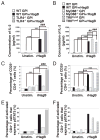
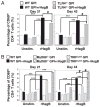
Recombinant hemagglutinin B (rHagB), a virulence factor of the periodontal pathogen Porphyromonas gingivalis, has been shown to induce protective immunity against bacterial infection. Furthermore, we have demonstrated that rHagB is a TLR4 agonist for dendritic cells. However, it is not known how rHagB dendritic cell stimulation affects the activation and differentiation of T cells. Therefore, we undertook the present study to examine the role of TLR4 signaling in shaping the CD4(+) T cell response following immunization of mice with rHagB. Immunization with this Ag resulted in the induction of specific CD4(+) T cells and Ab responses. In TLR4(-/-) and MyD88(-/-) but not Toll/IL-1R domain-containing adapter inducing IFN-β-deficient (TRIF(Lps2)) mice, there was an increase in the Th2 CD4(+) T cell subset, a decrease in the Th1 subset, and higher serum IgG(1)/IgG(2) levels of HagB-specific Abs compared with those in wild-type mice. These finding were accompanied by increased GATA-3 and Foxp3 expression and a decrease in the activation of CD4(+) T cells isolated from TLR4(-/-) and MyD88(-/-) mice. Interestingly, TLR4(-/-) CD4(+) T cells showed an increase in IL-2/STAT5 signaling. Whereas TRIF deficiency had minimal effects on the CD4(+) T cell response, it resulted in increased IFN-γ and IL-17 production by memory CD4(+) T cells. To our knowledge, these results demonstrate for the first time that TLR4 signaling, via the downstream MyD88 and TRIF molecules, exerts a differential regulation on the CD4(+) T cell response to HagB Ag. The gained insight from the present work will aid in designing better therapeutic strategies against P. gingivalis infection.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



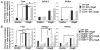


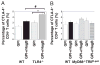
Similar articles
-
Aging and contribution of MyD88 and TRIF to expression of TLR pathway-associated genes following stimulation with Porphyromonas gingivalis.J Periodontal Res. 2015 Feb;50(1):89-102. doi: 10.1111/jre.12185. Epub 2014 May 24. J Periodontal Res. 2015. PMID: 24862405 Free PMC article.
-
MyD88 and TRIF synergistic interaction is required for TH1-cell polarization with a synthetic TLR4 agonist adjuvant.Eur J Immunol. 2013 Sep;43(9):2398-408. doi: 10.1002/eji.201243124. Epub 2013 Jul 3. Eur J Immunol. 2013. PMID: 23716300 Free PMC article.
-
Requirement of TLR4 and CD14 in dendritic cell activation by Hemagglutinin B from Porphyromonas gingivalis.Mol Immunol. 2009 Aug;46(13):2493-504. doi: 10.1016/j.molimm.2009.05.022. Epub 2009 Jun 21. Mol Immunol. 2009. PMID: 19540594 Free PMC article.
-
TLR4 biased small molecule modulators.Pharmacol Ther. 2021 Dec;228:107918. doi: 10.1016/j.pharmthera.2021.107918. Epub 2021 Jun 23. Pharmacol Ther. 2021. PMID: 34171331 Review.
-
Microbiota, Immune Subversion, and Chronic Inflammation.Front Immunol. 2017 Mar 13;8:255. doi: 10.3389/fimmu.2017.00255. eCollection 2017. Front Immunol. 2017. PMID: 28348558 Free PMC article. Review.
Cited by
-
Role of TLR2-dependent IL-10 production in the inhibition of the initial IFN-γ T cell response to Porphyromonas gingivalis.J Leukoc Biol. 2013 Jan;93(1):21-31. doi: 10.1189/jlb.0512220. Epub 2012 Oct 17. J Leukoc Biol. 2013. PMID: 23077245 Free PMC article.
-
Aging and contribution of MyD88 and TRIF to expression of TLR pathway-associated genes following stimulation with Porphyromonas gingivalis.J Periodontal Res. 2015 Feb;50(1):89-102. doi: 10.1111/jre.12185. Epub 2014 May 24. J Periodontal Res. 2015. PMID: 24862405 Free PMC article.
-
Unique Roles of TLR9- and MyD88-Dependent and -Independent Pathways in Adaptive Immune Responses to AAV-Mediated Gene Transfer.J Innate Immun. 2015;7(3):302-14. doi: 10.1159/000369273. Epub 2015 Jan 20. J Innate Immun. 2015. PMID: 25612611 Free PMC article.
-
Human beta defensin 3 alters matrix metalloproteinase production in human dendritic cells exposed to Porphyromonas gingivalis hemagglutinin B.J Periodontol. 2018 Mar;89(3):361-369. doi: 10.1002/JPER.17-0366. J Periodontol. 2018. PMID: 29543996 Free PMC article.
-
A unique host defense pathway: TRIF mediates both antiviral and antibacterial immune responses.Microbes Infect. 2013 Jan;15(1):1-10. doi: 10.1016/j.micinf.2012.10.011. Epub 2012 Oct 29. Microbes Infect. 2013. PMID: 23116944 Free PMC article. Review.
References
-
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. - PubMed
-
- Slots J, Bragd L, Wikström M, Dahlén G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontol. 1986;13:570–577. - PubMed
-
- López NJ. Occurrence of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia in progressive adult periodontitis. J Periodontol. 2000;71:948–954. - PubMed
-
- Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67(10, Suppl):1123–1137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

