More than a splicing code: integrating the role of RNA, chromatin and non-coding RNA in alternative splicing regulation
- PMID: 21497503
- PMCID: PMC6317717
- DOI: 10.1016/j.gde.2011.03.004
More than a splicing code: integrating the role of RNA, chromatin and non-coding RNA in alternative splicing regulation
Abstract
Large portions of the genome undergo alternative pre-mRNA splicing in often intricate patterns. Alternative splicing regulation requires extensive control mechanisms since errors can have deleterious consequences and may lead to developmental defects and disease. Recent work has identified a complex network of regulatory RNA elements which guide splicing decisions. In addition, the discovery that transcription and splicing are intimately coupled has opened up new directions into alternative splicing regulation. Work at the interface of chromatin and RNA biology has revealed unexpected molecular links between histone modifications, the transcription machinery, and non-coding RNAs (ncRNAs) in the determination of alternative splicing patterns.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
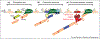

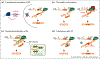
Similar articles
-
Connecting the dots: chromatin and alternative splicing in EMT.Biochem Cell Biol. 2016 Feb;94(1):12-25. doi: 10.1139/bcb-2015-0053. Epub 2015 Jul 7. Biochem Cell Biol. 2016. PMID: 26291837 Free PMC article. Review.
-
Connections between chromatin signatures and splicing.Wiley Interdiscip Rev RNA. 2013 Jan-Feb;4(1):77-91. doi: 10.1002/wrna.1142. Epub 2012 Oct 16. Wiley Interdiscip Rev RNA. 2013. PMID: 23074139 Review.
-
Chromatin: the final frontier in splicing regulation?Dev Cell. 2010 Mar 16;18(3):336-8. doi: 10.1016/j.devcel.2010.03.002. Dev Cell. 2010. PMID: 20230741 Free PMC article.
-
Where splicing joins chromatin.Nucleus. 2011 May-Jun;2(3):182-8. doi: 10.4161/nucl.2.3.15876. Nucleus. 2011. PMID: 21818411 Free PMC article. Review.
-
Regulation of alternative splicing by local histone modifications: potential roles for RNA-guided mechanisms.Nucleic Acids Res. 2014 Jan;42(2):701-13. doi: 10.1093/nar/gkt875. Epub 2013 Sep 29. Nucleic Acids Res. 2014. PMID: 24081581 Free PMC article. Review.
Cited by
-
Long lasting control of viral rebound with a new drug ABX464 targeting Rev - mediated viral RNA biogenesis.Retrovirology. 2015 Apr 9;12:30. doi: 10.1186/s12977-015-0159-3. Retrovirology. 2015. PMID: 25889234 Free PMC article.
-
Transgene Pyramiding of Salt Responsive Protein 3-1 (SaSRP3-1) and SaVHAc1 From Spartina alterniflora L. Enhances Salt Tolerance in Rice.Front Plant Sci. 2018 Sep 12;9:1304. doi: 10.3389/fpls.2018.01304. eCollection 2018. Front Plant Sci. 2018. PMID: 30258451 Free PMC article.
-
Differential pre-mRNA Splicing Alters the Transcript Diversity of Helitrons Between the Maize Inbred Lines.G3 (Bethesda). 2015 Jun 12;5(8):1703-11. doi: 10.1534/g3.115.018630. G3 (Bethesda). 2015. PMID: 26070844 Free PMC article.
-
Pre-mRNA splicing in disease and therapeutics.Trends Mol Med. 2012 Aug;18(8):472-82. doi: 10.1016/j.molmed.2012.06.006. Epub 2012 Jul 18. Trends Mol Med. 2012. PMID: 22819011 Free PMC article. Review.
-
TMEM2 Is a SOX4-Regulated Gene That Mediates Metastatic Migration and Invasion in Breast Cancer.Cancer Res. 2016 Sep 1;76(17):4994-5005. doi: 10.1158/0008-5472.CAN-15-2322. Epub 2016 Jun 21. Cancer Res. 2016. PMID: 27328729 Free PMC article.
References
-
-
Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB: Alternative isoform regulation in human tissue transcriptomes. Nature 2008.
One of the first genome-wide studies based on RNA deep-sequencing which revealed that large portions of the human genome are alternatively spliced in a highly tissue-specific fashion.
-
-
- Wang GS, Cooper TA: Splicing in disease: disruption of the splicing code and the decoding machinery. Nat Rev Genet 2007, 8:749–761. - PubMed
-
- Venables JP: Aberrant and alternative splicing in cancer. Cancer Res 2004, 64:7647–7654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

