Calcium-activated potassium channel KCa3.1 in lung dendritic cell migration
- PMID: 21493782
- PMCID: PMC3262686
- DOI: 10.1165/rcmb.2010-0514OC
Calcium-activated potassium channel KCa3.1 in lung dendritic cell migration
Abstract
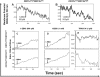
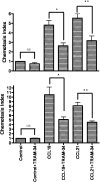
Migration to draining lymph nodes is a critical requirement for dendritic cells (DCs) to control T-cell-mediated immunity. The calcium-activated potassium channel KCa3.1 has been shown to be involved in regulating cell migration in multiple cell types. In this study, KCa3.1 expression and its functional role in lung DC migration were examined. Fluorescence-labeled antigen was intranasally delivered into mouse lungs to label lung Ag-carrying DCs. Lung CD11c(high)CD11b(low) and CD11c(low)CD11b(high) DCs from PBS-treated and ovalbumin (OVA)-sensitized mice were sorted using MACS and FACS. Indo-1 and DiBAC4(3) were used to measure intracellular Ca(2+) and membrane potential, respectively. The mRNA expression of KCa3.1 was examined using real-time PCR. Expression of KCa3.1 protein and CCR7 was measured using flow cytometry. Migration of two lung DC subsets to lymphatic chemokines was examined using TransWell in the absence or presence of the KCa3.1 blocker TRAM-34. OVA sensitization up-regulated mRNA and protein expression of KCa3.1 in lung DCs, with a greater response by the CD11c(high)CD11b(low) than CD11c(low)CD11b(high) DCs. Although KCa3.1 expression in Ag-carrying DCs was higher than that in non-Ag-carrying DCs in OVA-sensitized mice, the difference was not as prominent. However, Ag-carrying lung DCs expressed significantly higher CCR7 than non-Ag-carrying DCs. CCL19, CCL21, and KCa3.1 activator 1-EBIO induced an increase in intracellular calcium in both DC subsets. In addition, 1-EBIO-induced calcium increase was suppressed by TRAM-34. In vitro blockade of KCa3.1 with TRAM-34 impaired CCL19/CCL21-induced transmigration. In conclusion, KCa3.1 expression in lung DCs is up-regulated by OVA sensitization in both lung DC subsets, and KCa3.1 is involved in lung DC migration to lymphatic chemokines.
Figures


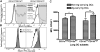
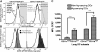

Similar articles
-
Fms-like tyrosine kinase 3 ligand regulates migratory pattern and antigen uptake of lung dendritic cell subsets in a murine model of allergic airway inflammation.J Immunol. 2009 Dec 1;183(11):7531-8. doi: 10.4049/jimmunol.0901341. Epub 2009 Nov 16. J Immunol. 2009. PMID: 19917684
-
Intermediate-conductance calcium-activated potassium channel KCa3.1 and chloride channel modulate chemokine ligand (CCL19/CCL21)-induced migration of dendritic cells.Transl Res. 2015 Jul;166(1):89-102. doi: 10.1016/j.trsl.2014.11.010. Epub 2014 Dec 20. Transl Res. 2015. PMID: 25583444 Free PMC article.
-
Downregulation of key early events in the mobilization of antigen-bearing dendritic cells by leukocyte immunoglobulin-like Receptor B4 in a mouse model of allergic pulmonary inflammation.PLoS One. 2013;8(2):e57007. doi: 10.1371/journal.pone.0057007. Epub 2013 Feb 19. PLoS One. 2013. PMID: 23431396 Free PMC article.
-
In vivo manipulation of dendritic cell migration and activation to elicit antitumour immunity.Novartis Found Symp. 2004;256:241-54; discussion 254-69. doi: 10.1002/0470856734.ch18. Novartis Found Symp. 2004. PMID: 15027495 Review.
-
Dendritic cell migration in inflammation and immunity.Cell Mol Immunol. 2021 Nov;18(11):2461-2471. doi: 10.1038/s41423-021-00726-4. Epub 2021 Jul 23. Cell Mol Immunol. 2021. PMID: 34302064 Free PMC article. Review.
Cited by
-
Cancer-Associated Intermediate Conductance Ca2+-Activated K⁺ Channel KCa3.1.Cancers (Basel). 2019 Jan 17;11(1):109. doi: 10.3390/cancers11010109. Cancers (Basel). 2019. PMID: 30658505 Free PMC article. Review.
-
SDF-1α and LPA modulate microglia potassium channels through rho gtpases to regulate cell morphology.Glia. 2013 Oct;61(10):1620-8. doi: 10.1002/glia.22543. Epub 2013 Jul 25. Glia. 2013. PMID: 23893870 Free PMC article.
-
KCa2 and KCa3.1 Channels in the Airways: A New Therapeutic Target.Biomedicines. 2023 Jun 21;11(7):1780. doi: 10.3390/biomedicines11071780. Biomedicines. 2023. PMID: 37509419 Free PMC article. Review.
-
Clinical view on the importance of dendritic cells in asthma.Expert Rev Clin Immunol. 2013 Oct;9(10):899-919. doi: 10.1586/1744666X.2013.837260. Expert Rev Clin Immunol. 2013. PMID: 24128155 Free PMC article. Review.
-
Targeting ion channels for the treatment of autoimmune neuroinflammation.Ther Adv Neurol Disord. 2013 Sep;6(5):322-36. doi: 10.1177/1756285613487782. Ther Adv Neurol Disord. 2013. PMID: 23997817 Free PMC article.
References
-
- Yanagihara S, Komura E, Nagafune J, Watarai H, Yamaguchi Y. EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. J Immunol 1998;161:3096–3102 - PubMed
-
- Chan VW, Kothakota S, Rohan MC, Panganiban-Lustan L, Gardner JP, Wachowicz MS, Winter JA, Williams LT. Secondary lymphoid-tissue chemokine (SLC) is chemotactic for mature dendritic cells. Blood 1999;93:3610–3616 - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta 2009;1793:933–940 - PubMed
-
- Hsu S, O'Connell PJ, Klyachko VA, Badminton MN, Thomson AW, Jackson MB, Clapham DE, Ahern GP. Fundamental Ca2+ signaling mechanisms in mouse dendritic cells: CRAC is the major Ca2+ entry pathway. J Immunol 2001;166:6126–6133 - PubMed
-
- Scandella E, Men Y, Legler DF, Gillessen S, Prikler L, Ludewig B, Groettrup M. CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2. Blood 2004;103:1595–1601 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

