Functional maturation of drug transporters in the developing, neonatal, and postnatal kidney
- PMID: 21493727
- PMCID: PMC3127534
- DOI: 10.1124/mol.110.070680
Functional maturation of drug transporters in the developing, neonatal, and postnatal kidney
Abstract
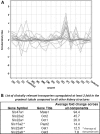
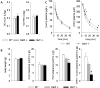
Because renal function in newborns is immature, the pharmacokinetics of drugs administered to neonates vary significantly from adult patients. The establishment of drug transport systems is a key process in the functional maturation of the nephron. However, a thorough examination of the expression of the main drug transporters in the kidney throughout all stages of development (embryonic, postnatal, and mature) has yet to be carried out, and the functional (physiological) impact is not well understood. Using time-series microarray data, we analyzed the temporal behavior of mRNA levels for a wide range of SLC and ABC transporters in the rodent kidney throughout a developmental time series. We find dynamic increases between the postnatal and mature stages of development for a number of transporters, including the proximal tubule-specific drug and organic anion transporters (OATs) OAT1 (SLC22a6) and OAT3 (SLC22a8). The OATs are the major multispecific basolateral drug, toxin, and metabolite transporters in the proximal tubule responsible for handling of many drugs, as well as the prototypical OAT substrate para-aminohippurate (PAH). We therefore performed specific in vivo pharmacokinetic analysis of the transport of PAH in postnatal and maturing rodent kidney. We show that there is a 4-fold increase in PAH clearance during this period. Clearance studies in Oat1 and Oat3 knockouts confirm that, as in the adult, Oat1 is the principle transporter of PAH in the postnatal kidney. The substantial differences observed supports the need for better understanding of pharmacokinetics in the newborn and juvenile kidney compared with the adult kidney at the basic and clinical level.
Figures



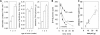
Similar articles
-
Dynamics of Organic Anion Transporter-Mediated Tubular Secretion during Postnatal Human Kidney Development and Maturation.Clin J Am Soc Nephrol. 2019 Apr 5;14(4):540-548. doi: 10.2215/CJN.10350818. Epub 2019 Mar 18. Clin J Am Soc Nephrol. 2019. PMID: 30885911 Free PMC article.
-
Rat and mouse differences in gender-predominant expression of organic anion transporter (Oat1-3; Slc22a6-8) mRNA levels.Drug Metab Dispos. 2004 Jun;32(6):620-5. doi: 10.1124/dmd.32.6.620. Drug Metab Dispos. 2004. PMID: 15155553
-
Analyses of coding region polymorphisms in apical and basolateral human organic anion transporter (OAT) genes [OAT1 (NKT), OAT2, OAT3, OAT4, URAT (RST)].Kidney Int. 2005 Oct;68(4):1491-9. doi: 10.1111/j.1523-1755.2005.00612.x. Kidney Int. 2005. PMID: 16164626
-
The multispecific organic anion transporter (OAT) family.Pflugers Arch. 2000 Jul;440(3):337-50. doi: 10.1007/s004240000297. Pflugers Arch. 2000. PMID: 10954321 Review.
-
Roles of organic anion transporters (OATs) in renal proximal tubules and their localization.Anat Sci Int. 2017 Mar;92(2):200-206. doi: 10.1007/s12565-016-0369-3. Epub 2016 Sep 10. Anat Sci Int. 2017. PMID: 27614971 Review.
Cited by
-
Potential implications of DMET ontogeny on the disposition of commonly prescribed drugs in neonatal and pediatric intensive care units.Expert Opin Drug Metab Toxicol. 2021 Mar;17(3):273-289. doi: 10.1080/17425255.2021.1858051. Epub 2021 Jan 20. Expert Opin Drug Metab Toxicol. 2021. PMID: 33256492 Free PMC article. Review.
-
The organic anion transporter (OAT) family: a systems biology perspective.Physiol Rev. 2015 Jan;95(1):83-123. doi: 10.1152/physrev.00025.2013. Physiol Rev. 2015. PMID: 25540139 Free PMC article. Review.
-
Characterization of the pattern of expression of Gas1 in the kidney during postnatal development in the rat.PLoS One. 2023 Apr 24;18(4):e0284816. doi: 10.1371/journal.pone.0284816. eCollection 2023. PLoS One. 2023. PMID: 37093844 Free PMC article.
-
Major glucuronide metabolites of testosterone are primarily transported by MRP2 and MRP3 in human liver, intestine and kidney.J Steroid Biochem Mol Biol. 2019 Jul;191:105350. doi: 10.1016/j.jsbmb.2019.03.027. Epub 2019 Apr 5. J Steroid Biochem Mol Biol. 2019. PMID: 30959153 Free PMC article.
-
The role of OAT2 (SLC22A7) in the cyclic nucleotide biokinetics of human erythrocytes.J Cell Physiol. 2018 Aug;233(8):5972-5980. doi: 10.1002/jcp.26409. Epub 2018 Feb 27. J Cell Physiol. 2018. PMID: 29244191 Free PMC article.
References
-
- Blackburn ST. (2007) Pituitary, adrenal, and thyroid function, in Maternal, Fetal & Neonatal Physiology: a Clinical Perspective, p. 669–699, Saunders, St. Louis, MO
-
- Bräunlich H. (1984) Postnatal development of kidney function in rats receiving thyroid hormones. Exp Clin Endocrinol 83:243–250 - PubMed
-
- Buist SC, Cherrington NJ, Choudhuri S, Hartley DP, Klaassen CD. (2002) Gender-specific and developmental influences on the expression of rat organic anion transporters. J Pharmacol Exp Ther 301:145–151 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
