Topography of lipid droplet-associated proteins: insights from freeze-fracture replica immunogold labeling
- PMID: 21490801
- PMCID: PMC3068475
- DOI: 10.1155/2011/409371
Topography of lipid droplet-associated proteins: insights from freeze-fracture replica immunogold labeling
Abstract
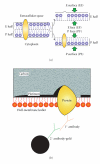
Lipid droplets are not merely storage depots for superfluous intracellular lipids in times of hyperlipidemic stress, but metabolically active organelles involved in cellular homeostasis. Our concepts on the metabolic functions of lipid droplets have come from studies on lipid droplet-associated proteins. This realization has made the study of proteins, such as PAT family proteins, caveolins, and several others that are targeted to lipid droplets, an intriguing and rapidly developing area of intensive inquiry. Our existing understanding of the structure, protein organization, and biogenesis of the lipid droplet has relied heavily on microscopical techniques that lack resolution and the ability to preserve native cellular and protein composition. Freeze-fracture replica immunogold labeling overcomes these disadvantages and can be used to define at high resolution the precise location of lipid droplet-associated proteins. In this paper illustrative examples of how freeze-fracture immunocytochemistry has contributed to our understanding of the spatial organization in the membrane plane and function of PAT family proteins and caveolin-1 are presented. By revisiting the lipid droplet with freeze-fracture immunocytochemistry, new perspectives have emerged which challenge prevailing concepts of lipid droplet biology and may hopefully provide a timely impulse for many ongoing studies.
Figures









Similar articles
-
Compartmentalization of proteins in lipid droplet biogenesis.Biochim Biophys Acta. 2009 Jun;1791(6):408-18. doi: 10.1016/j.bbalip.2008.12.001. Epub 2008 Dec 11. Biochim Biophys Acta. 2009. PMID: 19118639 Review.
-
Lipids partition caveolin-1 from ER membranes into lipid droplets: updating the model of lipid droplet biogenesis.FASEB J. 2004 May;18(7):866-8. doi: 10.1096/fj.03-0782fje. Epub 2004 Mar 4. FASEB J. 2004. PMID: 15001554
-
Lipid droplets gain PAT family proteins by interaction with specialized plasma membrane domains.J Biol Chem. 2005 Jul 15;280(28):26330-8. doi: 10.1074/jbc.M413312200. Epub 2005 May 16. J Biol Chem. 2005. PMID: 15897193
-
PAT family proteins pervade lipid droplet cores.J Lipid Res. 2005 Jun;46(6):1331-8. doi: 10.1194/jlr.M400323-JLR200. Epub 2005 Mar 1. J Lipid Res. 2005. PMID: 15741656
-
Freeze-fracture cytochemistry in cell biology.Methods Cell Biol. 2008;88:181-204. doi: 10.1016/S0091-679X(08)00411-1. Methods Cell Biol. 2008. PMID: 18617035 Review.
Cited by
-
New Method for Quantitation of Lipid Droplet Volume From Light Microscopic Images With an Application to Determination of PAT Protein Density on the Droplet Surface.J Histochem Cytochem. 2018 Jun;66(6):447-465. doi: 10.1369/0022155417753573. Epub 2018 Jan 23. J Histochem Cytochem. 2018. PMID: 29361239 Free PMC article.
-
HIV protease inhibitors disrupt lipid metabolism by activating endoplasmic reticulum stress and inhibiting autophagy activity in adipocytes.PLoS One. 2013;8(3):e59514. doi: 10.1371/journal.pone.0059514. Epub 2013 Mar 22. PLoS One. 2013. PMID: 23533630 Free PMC article.
-
The dynamic roles of intracellular lipid droplets: from archaea to mammals.Protoplasma. 2012 Jul;249(3):541-85. doi: 10.1007/s00709-011-0329-7. Epub 2011 Oct 15. Protoplasma. 2012. PMID: 22002710 Review.
-
ER Stress and Lipid Metabolism in Adipocytes.Biochem Res Int. 2012;2012:312943. doi: 10.1155/2012/312943. Epub 2012 Feb 12. Biochem Res Int. 2012. PMID: 22400114 Free PMC article.
-
Synchrotron multimodal imaging in a whole cell reveals lipid droplet core organization.J Synchrotron Radiat. 2020 May 1;27(Pt 3):772-778. doi: 10.1107/S1600577520003847. Epub 2020 Apr 23. J Synchrotron Radiat. 2020. PMID: 32381780 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

