Oral tolerance
- PMID: 21488901
- PMCID: PMC3296283
- DOI: 10.1111/j.1600-065X.2011.01017.x
Oral tolerance
Abstract
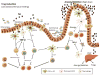
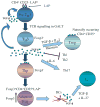
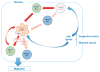
The gut-associated lymphoid tissue is the largest immune organ in the body and is the primary route by which we are exposed to antigens. Tolerance induction is the default immune pathway in the gut, and the type of tolerance induced relates to the dose of antigen fed: anergy/deletion (high dose) or regulatory T-cell (Treg) induction (low dose). Conditioning of gut dendritic cells (DCs) by gut epithelial cells and the gut flora, which itself has a major influence on gut immunity, induces CD103(+) retinoic acid-dependent DC that induces Tregs. A number of Tregs are induced at mucosal surfaces. Th3 type Tregs are transforming growth factor-β dependent and express latency-associated peptide (LAP) on their surface and were discovered in the context of oral tolerance. Tr1 type Tregs (interleukin-10 dependent) are induced by nasal antigen and forkhead box protein 3(+) iTregs are induced by oral antigen and by oral administration of aryl hydrocarbon receptor ligands. Oral or nasal antigen ameliorates autoimmune and inflammatory diseases in animal models by inducing Tregs. Furthermore, anti-CD3 monoclonal antibody is active at mucosal surfaces and oral or nasal anti-CD3 monoclonal antibody induces LAP(+) Tregs that suppresses animal models (experimental autoimmune encephalitis, type 1 and type 2 diabetes, lupus, arthritis, atherosclerosis) and is being tested in humans. Although there is a large literature on treatment of animal models by mucosal tolerance and some positive results in humans, this approach has yet to be translated to the clinic. The successful translation will require defining responsive patient populations, validating biomarkers to measure immunologic effects, and using combination therapy and immune adjuvants to enhance Treg induction. A major avenue being investigated for the treatment of autoimmunity is the induction of Tregs and mucosal tolerance represents a non-toxic, physiologic approach to reach this goal.
© 2011 John Wiley & Sons A/S.
Figures

Similar articles
-
Oral tolerance: therapeutic implications for autoimmune diseases.Clin Dev Immunol. 2006 Jun-Dec;13(2-4):143-57. doi: 10.1080/17402520600876804. Clin Dev Immunol. 2006. PMID: 17162357 Free PMC article. Review.
-
Cellular Components and Mechanisms of Oral Tolerance Induction.Crit Rev Immunol. 2018;38(3):207-231. doi: 10.1615/CritRevImmunol.2018026181. Crit Rev Immunol. 2018. PMID: 30004858 Review.
-
Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells.Microbes Infect. 2001 Sep;3(11):947-54. doi: 10.1016/s1286-4579(01)01456-3. Microbes Infect. 2001. PMID: 11564443 Review.
-
Oral tolerance: elucidation of mechanisms and application to treatment of autoimmune diseases.Biopolymers. 1997;43(4):323-35. doi: 10.1002/(SICI)1097-0282(1997)43:4<323::AID-BIP5>3.0.CO;2-X. Biopolymers. 1997. PMID: 9316395 Review.
-
Intranasal immunization with heat shock protein 60 induces CD4(+) CD25(+) GARP(+) and type 1 regulatory T cells and inhibits early atherosclerosis.Clin Exp Immunol. 2016 Mar;183(3):452-68. doi: 10.1111/cei.12726. Epub 2015 Nov 24. Clin Exp Immunol. 2016. PMID: 26452441 Free PMC article.
Cited by
-
Distinct functions and transcriptional signatures in orally induced regulatory T cell populations.Front Immunol. 2023 Oct 26;14:1278184. doi: 10.3389/fimmu.2023.1278184. eCollection 2023. Front Immunol. 2023. PMID: 37954612 Free PMC article.
-
Antigen-based vs. systemic immunomodulation in type 1 diabetes: the pros and cons.Islets. 2013 Mar-Apr;5(2):53-66. doi: 10.4161/isl.24785. Epub 2013 Mar 1. Islets. 2013. PMID: 23648893 Free PMC article. Review.
-
Plant-based oral tolerance to hemophilia therapy employs a complex immune regulatory response including LAP+CD4+ T cells.Blood. 2015 Apr 9;125(15):2418-27. doi: 10.1182/blood-2014-08-597070. Epub 2015 Feb 19. Blood. 2015. PMID: 25700434 Free PMC article.
-
Gut Microbiota and Mucosal Immunity in the Neonate.Med Sci (Basel). 2018 Jul 17;6(3):56. doi: 10.3390/medsci6030056. Med Sci (Basel). 2018. PMID: 30018263 Free PMC article. Review.
-
IgA antibody production by intrarectal immunization of mice using recombinant major capsid protein of hamster polyomavirus.Eur J Microbiol Immunol (Bp). 2012 Sep;2(3):231-8. doi: 10.1556/EuJMI.2.2012.3.9. Epub 2012 Sep 10. Eur J Microbiol Immunol (Bp). 2012. PMID: 24688770 Free PMC article.
References
-
- Moog F. The lining of the small intestine. Sci Am. 1981;245:154–158. 160, 162. et passiom. - PubMed
-
- Mestecky J, McGhee JR. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. - PubMed
-
- Brandtzaeg P. Development and basic mechanisms of human gut immunity. Nutr Rev. 1998;56:S5–S18. - PubMed
-
- Macfarlane GT, Macfarlane S. Human colonic microbiota: ecology, physiology and metabolic potential of intestinal bacteria. Scand J Gastroenterol Suppl. 1997;222:3–9. - PubMed
-
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

